Answered step by step
Verified Expert Solution
Question
1 Approved Answer
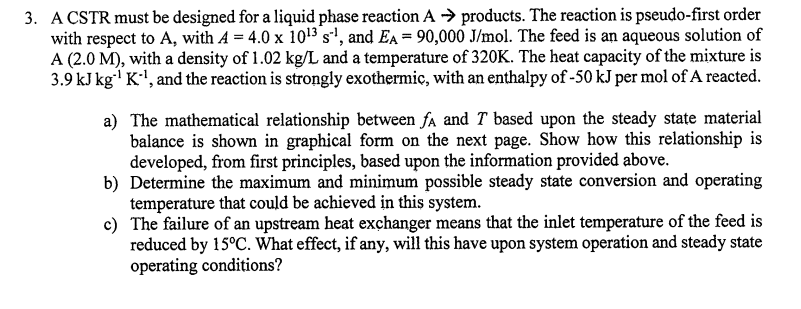
A CSTR must be designed for a liquid phase reaction A products. The reaction is pseudo - first order with respect to A , with
A CSTR must be designed for a liquid phase reaction products. The reaction is pseudofirst order
with respect to with and The feed is an aqueous solution of
with a density of and a temperature of The heat capacity of the mixture is
and the reaction is strongly exothermic, with an enthalpy of per mol of A reacted.
a The mathematical relationship between and based upon the steady state material
balance is shown in graphical form on the next page. Show how this relationship is
developed, from first principles, based upon the information provided above.
b Determine the maximum and minimum possible steady state conversion and operating
temperature that could be achieved in this system.
c The failure of an upstream heat exchanger means that the inlet temperature of the feed is
reduced by What effect, if any, will this have upon system operation and steady state
operating conditions?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


