Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A cylinder of volume 0.270 m contains 11.1 mol of neon gas at 19.8C. Assume neon behaves as an ideal gas. (a) What is
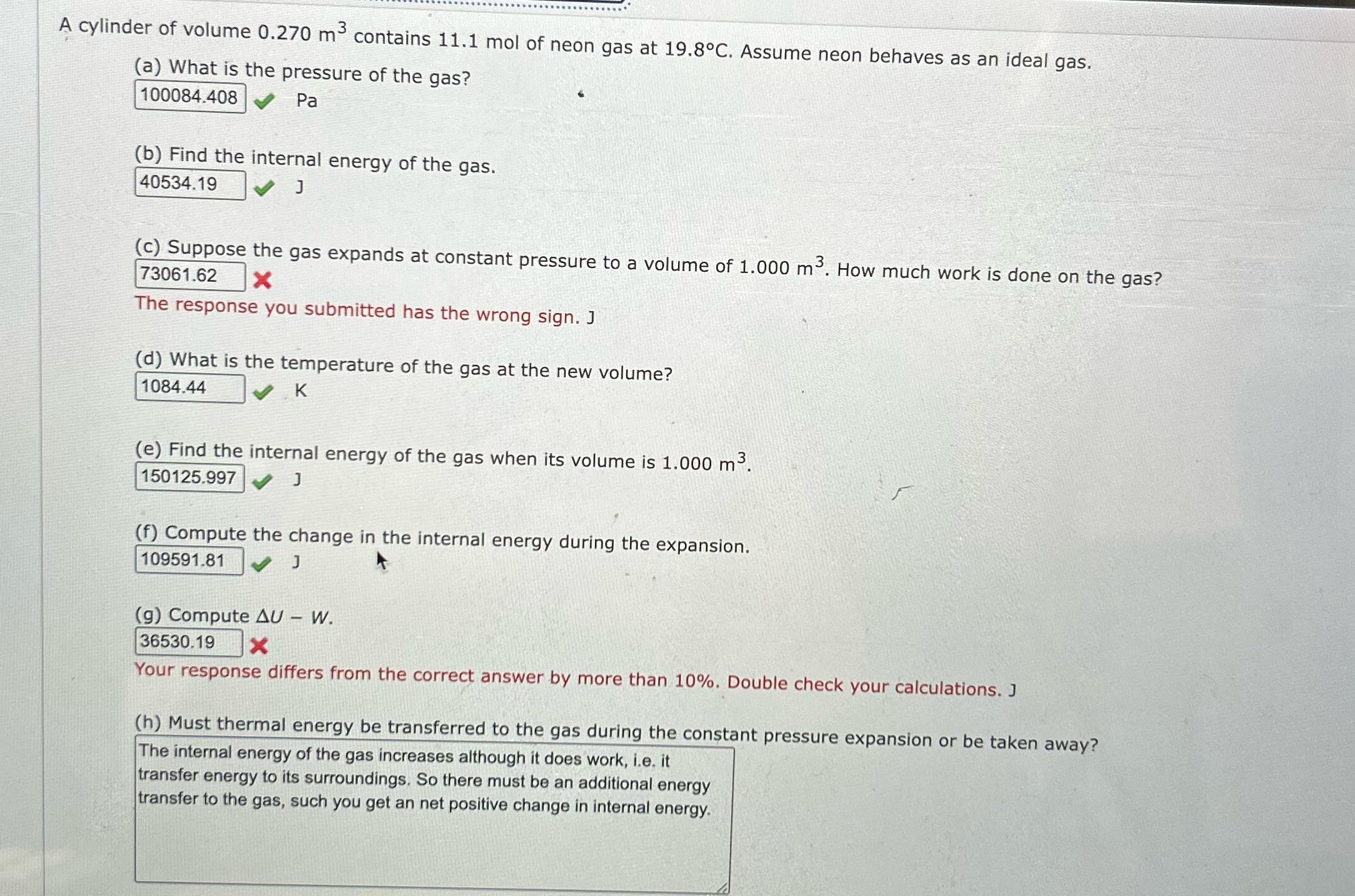
A cylinder of volume 0.270 m contains 11.1 mol of neon gas at 19.8C. Assume neon behaves as an ideal gas. (a) What is the pressure of the gas? 100084.408 Pa (b) Find the internal energy of the gas. 40534.19 J (c) Suppose the gas expands at constant pressure to a volume of 1.000 m. How much work is done on the gas? 73061.62 X The response you submitted has the wrong sign. J (d) What is the temperature of the gas at the new volume? 1084.44 K (e) Find the internal energy of the gas when its volume is 1.000 m. 150125.997 J (f) Compute the change in the internal energy during the expansion. 109591.81 J (g) Compute AU - W. 36530.19 Your response differs from the correct answer by more than 10%. Double check your calculations. J (h) Must thermal energy be transferred to the gas during the constant pressure expansion or be taken away? The internal energy of the gas increases although it does work, i.e. it transfer energy to its surroundings. So there must be an additional energy transfer to the gas, such you get an net positive change in internal energy.
Step by Step Solution
★★★★★
3.42 Rating (152 Votes )
There are 3 Steps involved in it
Step: 1
Heres the corrected solution with explanations a Pressure of the gas We can use the ideal gas law PV ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


