Answered step by step
Verified Expert Solution
Question
1 Approved Answer
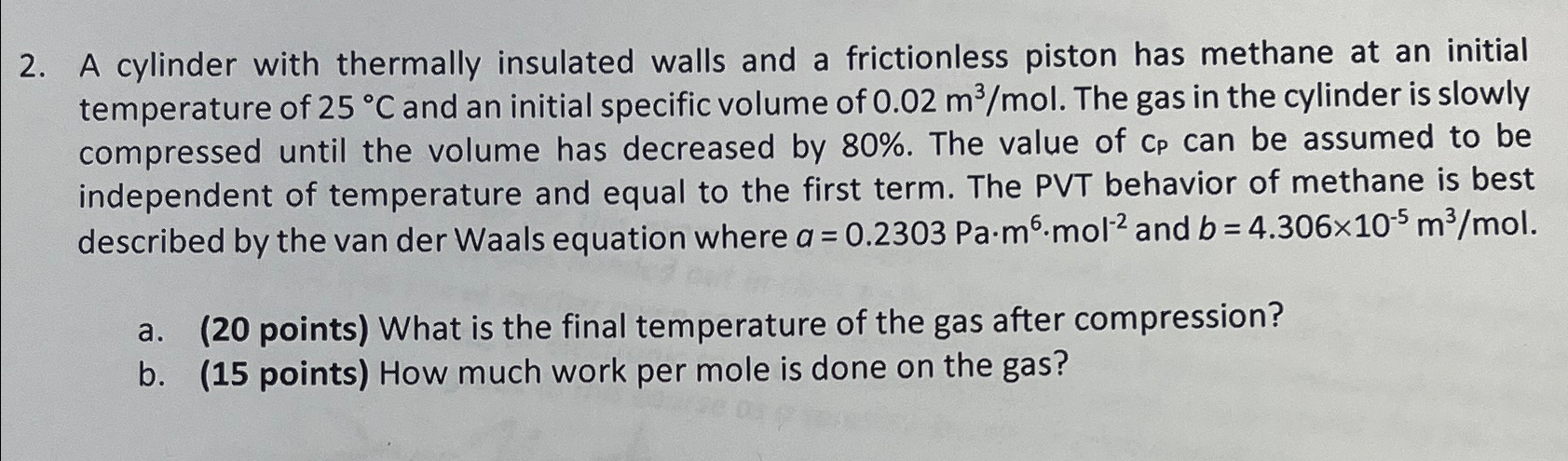
A cylinder with thermally insulated walls and a frictionless piston has methane at an initial temperature of 2 5 C and an initial specific volume
A cylinder with thermally insulated walls and a frictionless piston has methane at an initial temperature of and an initial specific volume of The gas in the cylinder is slowly compressed until the volume has decreased by The value of be assumed to be independent of temperature and equal to the first term. The PVT behavior of methane is best described by the van der Waals equation where and
a points What is the final temperature of the gas after compression?
b points How much work per mole is done on the gas?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


