Answered step by step
Verified Expert Solution
Question
1 Approved Answer
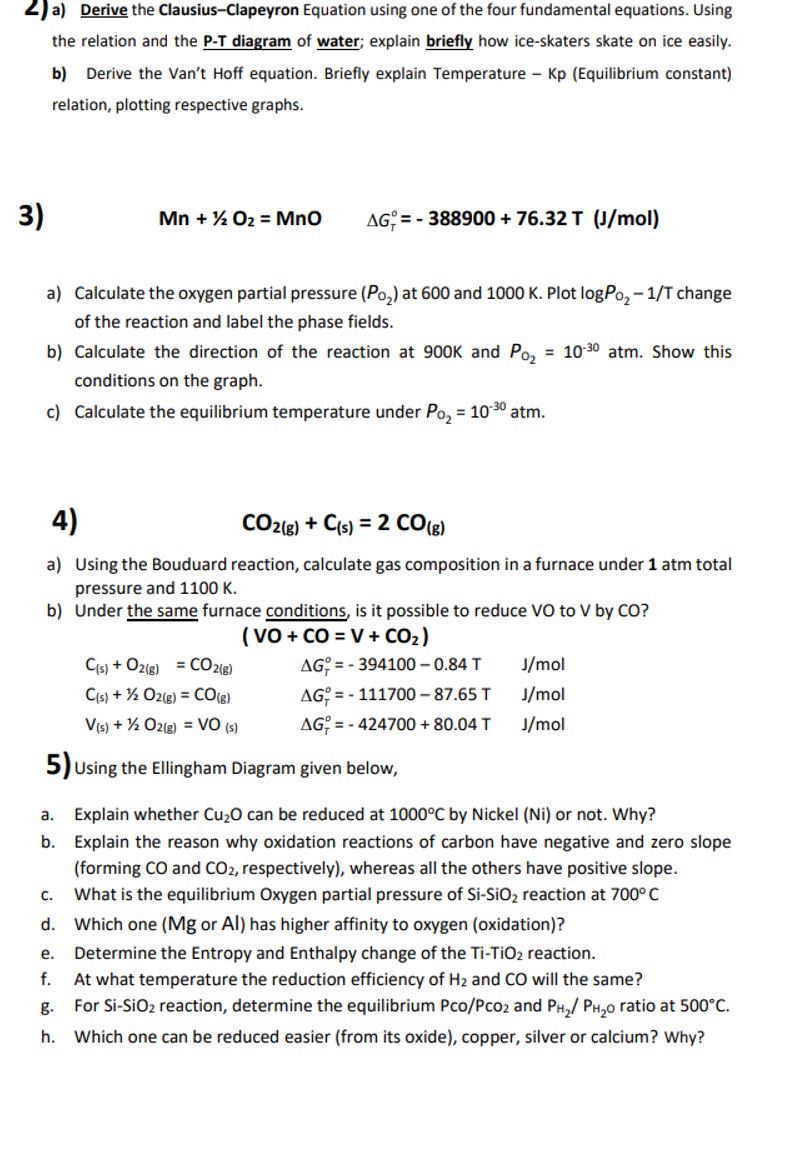
a ) Derive the Clausius - Clapeyron Equation using one of the four fundamental equations. Using the relation and the P - T diagram of
a Derive the ClausiusClapeyron Equation using one of the four fundamental equations. Using
the relation and the T diagram of water; explain briefly how iceskaters skate on ice easily.
b Derive the Van't Hoff equation. Briefly explain Temperature Kp Equilibrium constant
relation, plotting respective graphs.
MnO,
a Calculate the oxygen partial pressure at and Plot change
of the reaction and label the phase fields.
b Calculate the direction of the reaction at and atm. Show this
conditions on the graph.
c Calculate the equilibrium temperature under atm.
a Using the Bouduard reaction, calculate gas composition in a furnace under atm total
pressure and
b Under the same furnace conditions, is it possible to reduce VO to by
Using the Ellingham Diagram given below,
a Explain whether can be reduced at by Nickel Ni or not. Why?
b Explain the reason why oxidation reactions of carbon have negative and zero slope
forming and respectively whereas all the others have positive slope.
d Which one Mg or has higher affinity to oxygen oxidation
e Determine the Entropy and Enthalpy change of the reaction.
f At what temperature the reduction efficiency of and will the same?
g For reaction, determine the equilibrium and ratio at
h Which one can be reduced easier from its oxide copper, silver or calcium? W
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


