Answered step by step
Verified Expert Solution
Question
1 Approved Answer
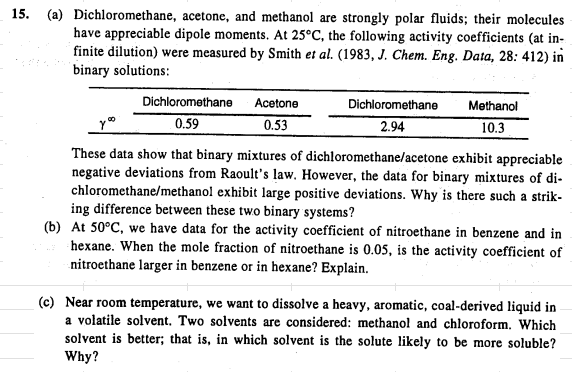
( a ) Dichloromethane, acetone, and methanol are strongly polar fluids; their molecules have appreciable dipole moments. At 2 5 C , the following activity
a Dichloromethane, acetone, and methanol are strongly polar fluids; their molecules
have appreciable dipole moments. At the following activity coefficients at in
finite dilution were measured by Smith et al J Chem. Eng. Data, : in
binary solutions:
These data show that binary mixtures of dichloromethaneacetone exhibit appreciable
negative deviations from Raoult's law. However, the data for binary mixtures of di
chloromethanemethanol exhibit large positive deviations. Why is there such a strik
ing difference between these two binary systems?
b At we have data for the activity coefficient of nitroethane in benzene and in
hexane. When the mole fraction of nitroethane is is the activity coefficient of
nitroethane larger in benzene or in hexane? Explain.
c Near room temperature, we want to dissolve a heavy, aromatic, coalderived liquid in
a volatile solvent. Two solvents are considered: methanol and chloroform. Which
solvent is better; that is in which solvent is the solute likely to be more soluble?
Why?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


