Question
A distillation column separates 10,000 lb/hr of a liquid solution at 70F containing 40% benzene and 60% chloroform. The liquid product distilled from the column
A distillation column separates 10,000 lb/hr of a liquid solution at 70F containing 40% benzene and 60% chloroform. The liquid product distilled from the column is 99.5% benzene while the residue product (reheater stream) contains 1% benzene. The condenser uses water which enters at 60F and exits at 140, and the reheater uses steam saturated at 280F. The reflux reaction (the ratio of the distillate liquid returned to the column to the distillate product extracted) is 6 to 1. Assuming that the condenser and reheater operate at 1 atm; also that the calculated temperature for the condenser is 178F; for the reheater, 268F; and that the fraction of benzene determined in the reheater steam is 3.9% by weight (5.5 mole %). Calculate: (a) Pounds of distillate product and residue per hour. b) Pounds of reflux per hour c) Pounds of liquid entering the superheater and vapor leaving the superheater per hour. d) Pounds of steam and cooling water consumed per hour. f) Recalculate if distillation column feed data differ 5%. For composition consider that 5% variation for 40% of the benzene (the rest is chloroform) and compare to the original problem.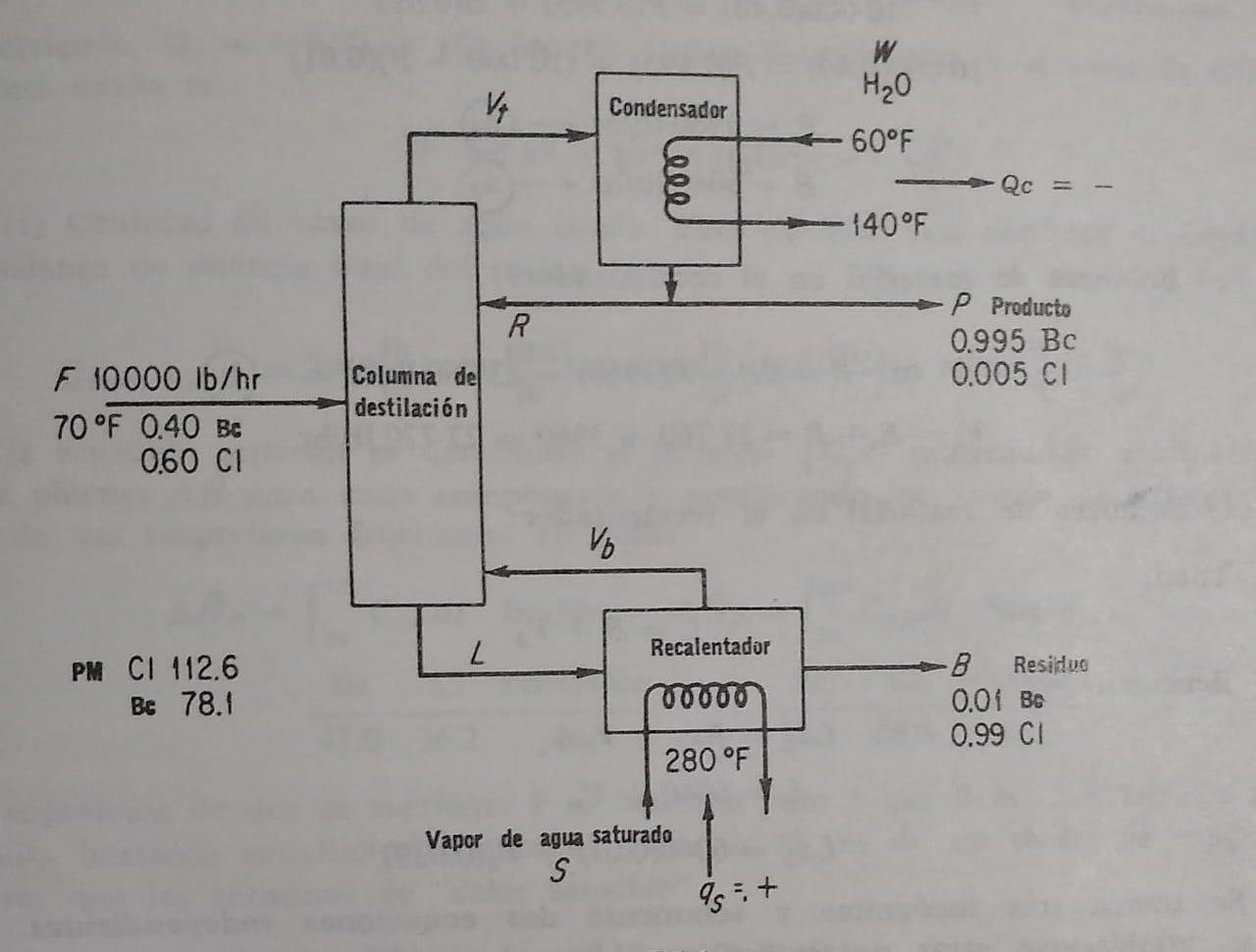
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


