Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A distillation system with a still pot plus a column with one equilibrium stage is used to batch distill 1 . 0 kmol of a
A distillation system with a still pot plus a column with one equilibrium stage is used to batch distill kmol of a mol methanol and mol water feed. A total condenser is used. The final bottoms concentration is mol methanol. Pressure is kPa. Reflux is a saturated liquid, and The equilibrium curve is in Fig. Find and
Fig. y vs x diagram for methanolwater at kPa.
part B
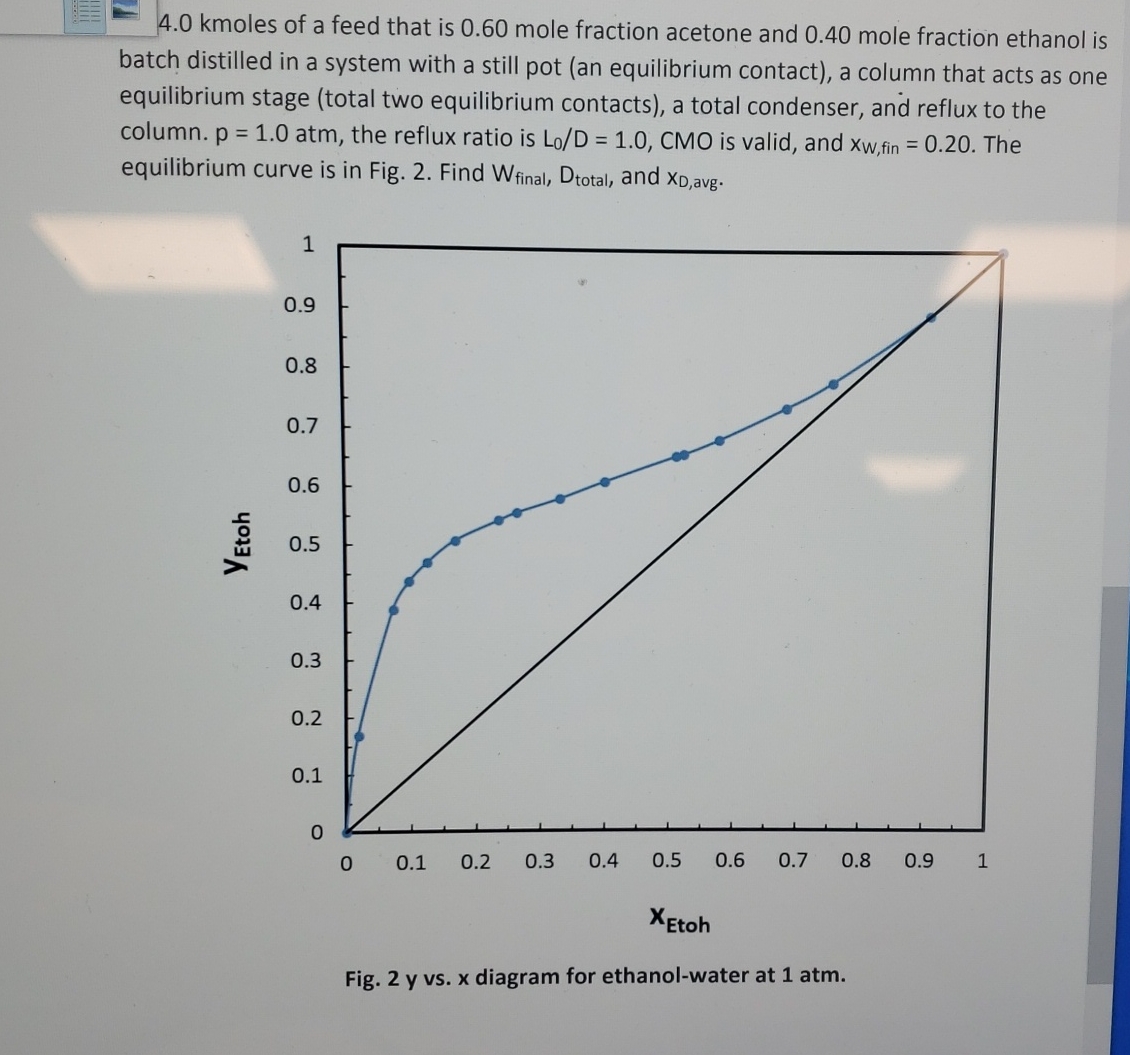
kmoles of a feed that is mole fraction acetone and mole fraction ethanol is batch distilled in a system with a still pot an equilibrium contact a column that acts as one equilibrium stage total two equilibrium contacts a total condenser, and reflux to the column. atm, the reflux ratio is CMO is valid, and fin The equilibrium curve is in Fig. Find and
Fig. y vs diagram for ethanolwater at atm.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


