Answered step by step
Verified Expert Solution
Question
1 Approved Answer
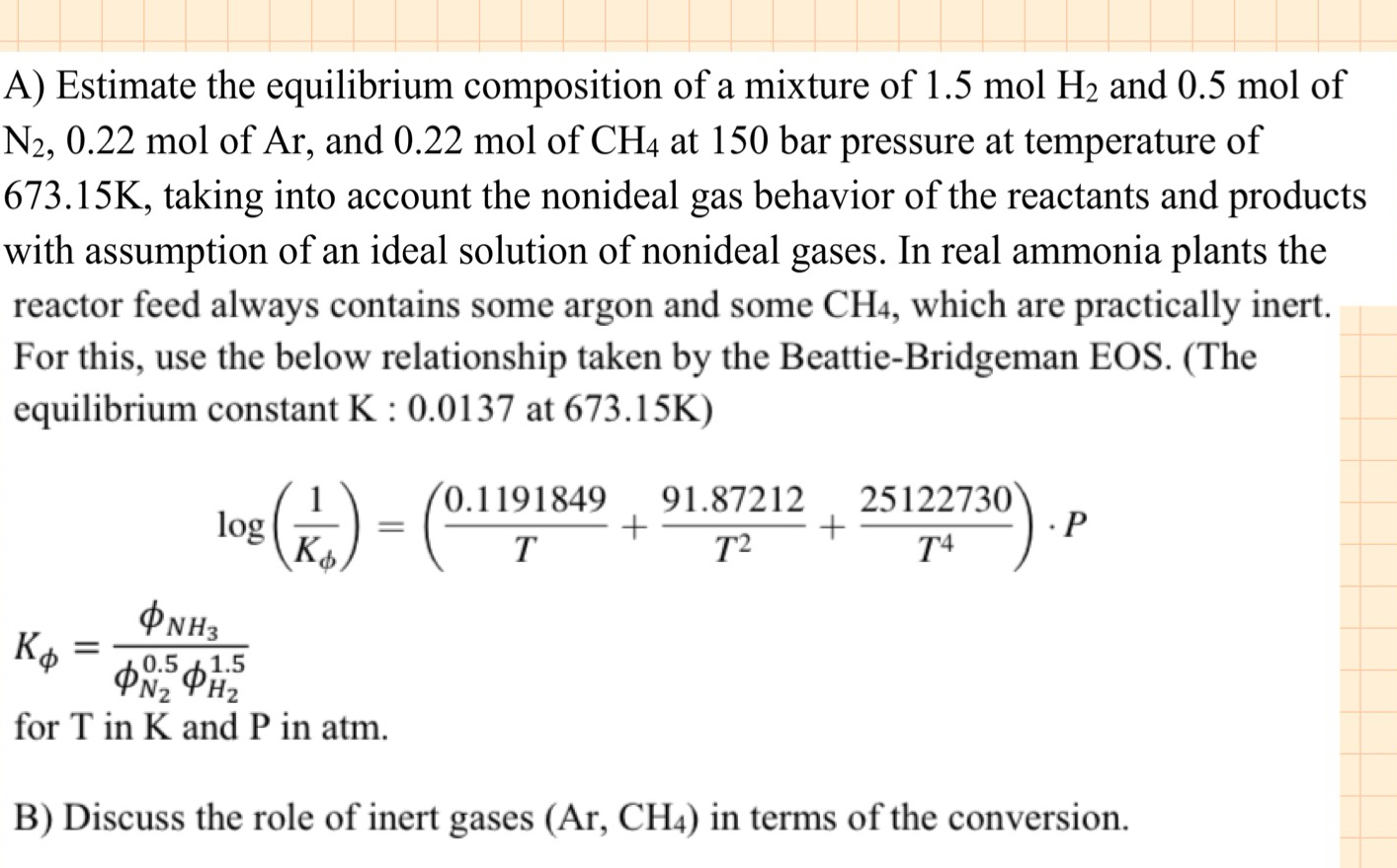
A ) Estimate the equilibrium composition of a mixture of 1 . 5 m o l H 2 and 0 . 5 mol of N
A Estimate the equilibrium composition of a mixture of and mol of mol of and at bar pressure at temperature of taking into account the nonideal gas behavior of the reactants and products with assumption of an ideal solution of nonideal gases. In real ammonia plants the reactor feed always contains some argon and some which are practically inert. For this, use the below relationship taken by the BeattieBridgeman EOS. The equilibrium constant : at
for in and in atm.
B Discuss the role of inert gases in terms of the conversion.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


