Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A first order reaction is taking place inside a porous catalyst. Assume dilute concentrations and neglect any variations in the axial (x) direction. Drive an
A first order reaction is taking place inside a porous catalyst. Assume dilute concentrations and neglect any variations in the axial (x) direction. Drive an equation for both the internal and overall effectiveness factors for the rectangular porous slab shown below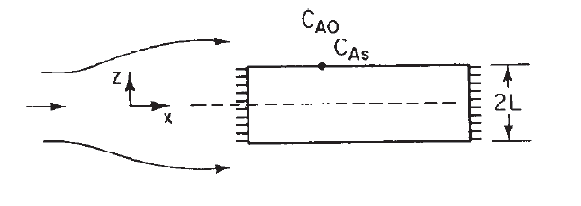
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


