Answered step by step
Verified Expert Solution
Question
1 Approved Answer
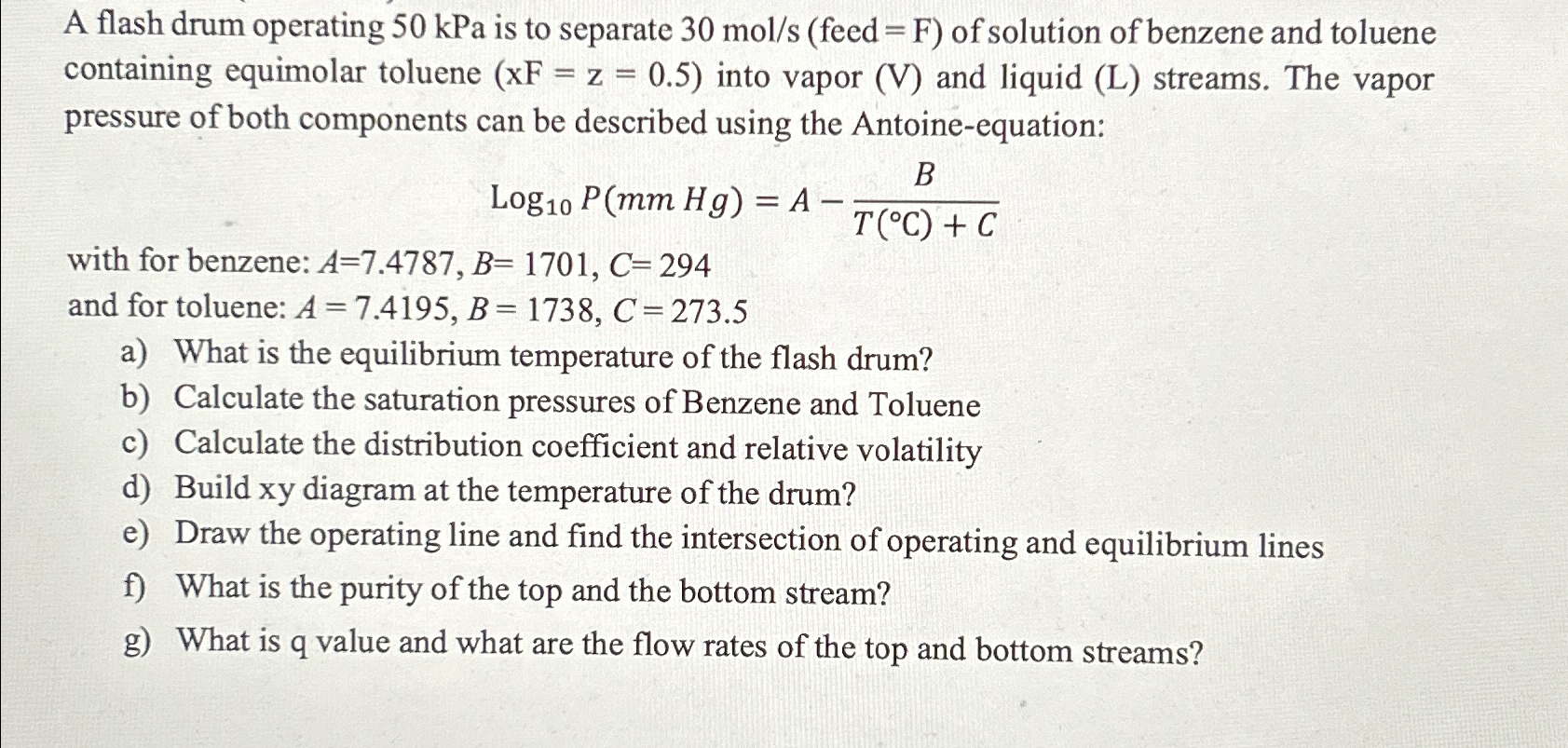
A flash drum operating 5 0 kPa is to separate ) of solution of benzene and toluene containing equimolar toluene ) = z = (
A flash drum operating kPa is to separate of solution of benzene and toluene containing equimolar toluene into vapor and liquid streams. The vapor pressure of both components can be described using the Antoineequation:
with for benzene:
and for toluene:
a What is the equilibrium temperature of the flash drum?
b Calculate the saturation pressures of Benzene and Toluene
c Calculate the distribution coefficient and relative volatility
d Build xy diagram at the temperature of the drum?
e Draw the operating line and find the intersection of operating and equilibrium lines
f What is the purity of the top and the bottom stream?
g What is q value and what are the flow rates of the top and bottom streams?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


