Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A flat plate of solid carbon is being burned in the presence of pure oxygen according to the reaction 2C(s) +20(g) CO(g)+co,(9) + Molecular diffusion
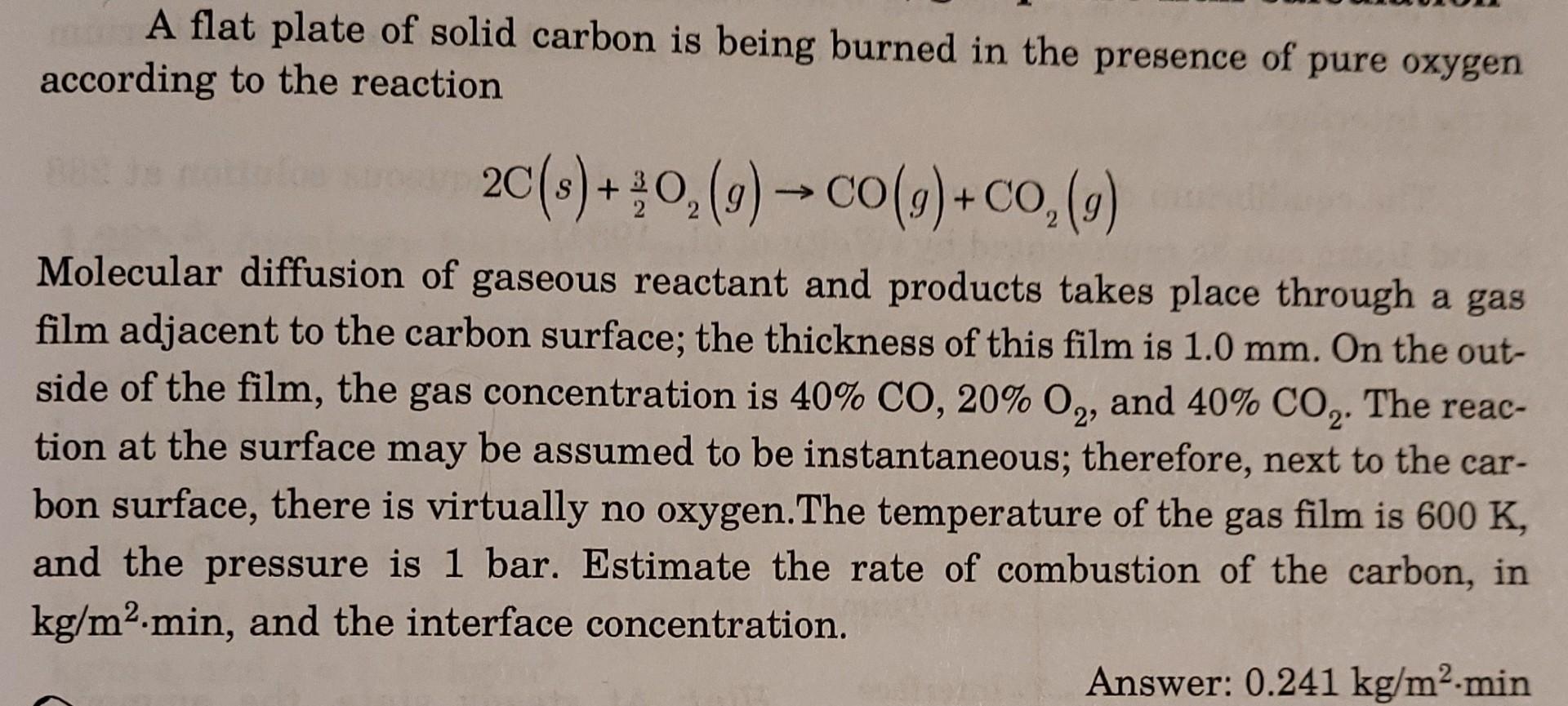
A flat plate of solid carbon is being burned in the presence of pure oxygen according to the reaction 2C(s) +20(g) CO(g)+co,(9) + Molecular diffusion of gaseous reactant and products takes place through a gas film adjacent to the carbon surface; the thickness of this film is 1.0 mm. On the out- side of the film, the gas concentration is 40% CO, 20% O2, and 40% CO2. The reac- tion at the surface may be assumed to be instantaneous; therefore, next to the car- bon surface, there is virtually no oxygen.The temperature of the gas film is 600 K, and the pressure is 1 bar. Estimate the rate of combustion of the carbon, in kg/m2.min, and the interface concentration. Answer: 0.241 kg/m.min
Step by Step Solution
There are 3 Steps involved in it
Step: 1
To estimate the rate of combustion of the carbon and the interface concentration we will follow thes...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


