Answered step by step
Verified Expert Solution
Question
1 Approved Answer
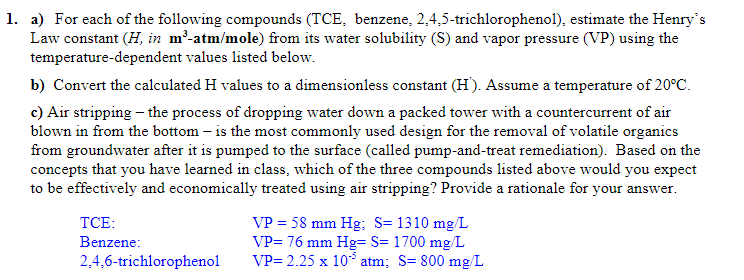
a ) For each of the following compounds ( TCE , benzene, 2 , 4 , 5 - trichlorophenol ) , estimate the Henry's Law
a For each of the following compounds TCE benzene, trichlorophenol estimate the Henry's
Law constant in atmmole from its water solubility S and vapor pressure VP using the
temperaturedependent values listed below.
b Convert the calculated values to a dimensionless constant Assume a temperature of
c Air stripping the process of dropping water down a packed tower with a countercurrent of air
blown in from the bottom is the most commonly used design for the removal of volatile organics
from groundwater after it is pumped to the surface called pumpandtreat remediation Based on the
concepts that you have learned in class, which of the three compounds listed above would you expect
to be effectively and economically treated using air stripping? Provide a rationale for your answer.
TCE:
Benzene:
;
trichlorophenol
atm;
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


