Answered step by step
Verified Expert Solution
Question
1 Approved Answer
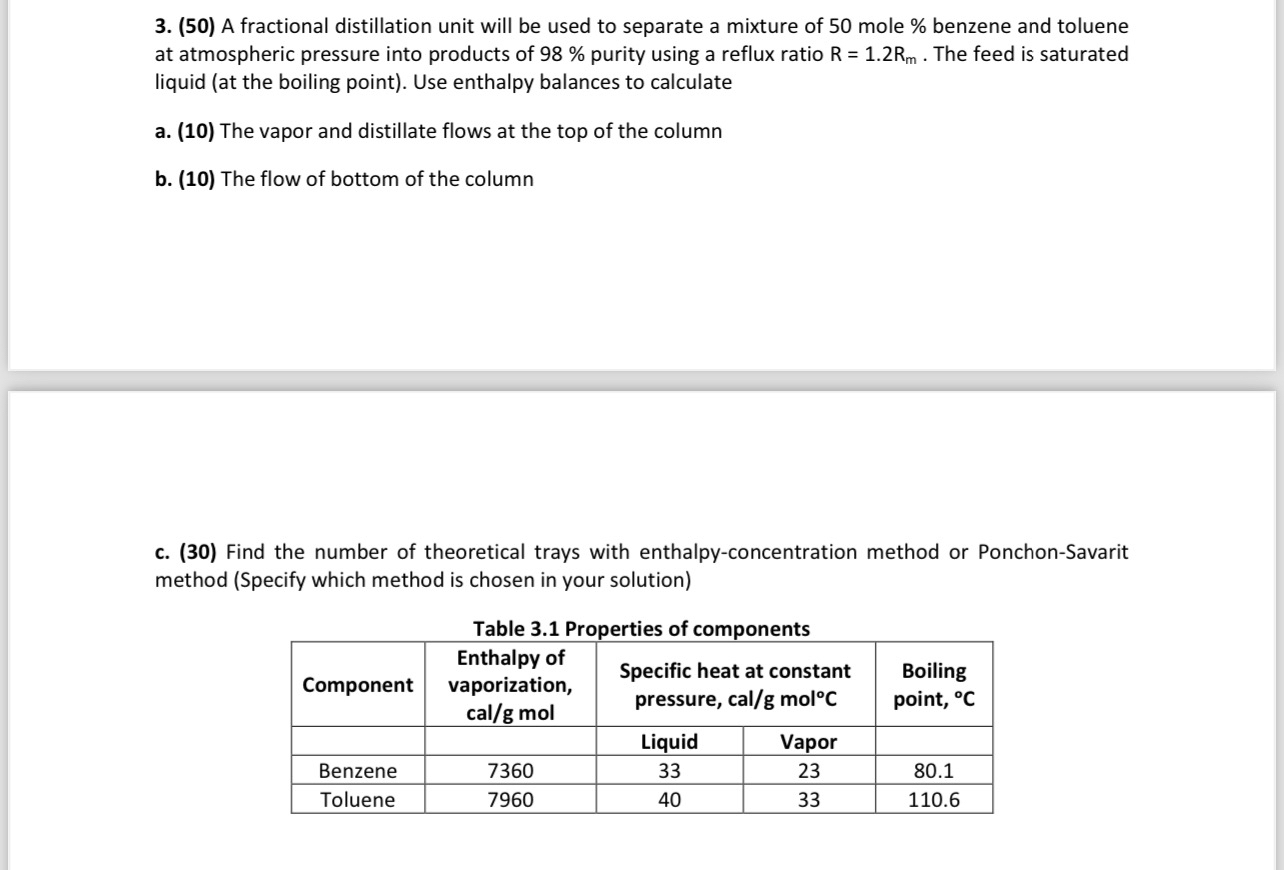
A fractional distillation unit will be used to separate a mixture of 5 0 mole % benzene and toluene at atmospheric pressure into products of
A fractional distillation unit will be used to separate a mixture of mole benzene and toluene at atmospheric pressure into products of purity using a reflux ratio The feed is saturated liquid at the boiling point Use enthalpy balances to calculate
a The vapor and distillate flows at the top of the column
b The flow of bottom of the column
c Find the number of theoretical trays with enthalpyconcentration method or PonchonSavarit method Specify which method is chosen in your solution
Table Properties of components
tableComponenttableEnthalpy ofvaporizationcalg moltableSpecific heat at constantpressure calg mol
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


