Question
A fractionation tower operating at 101.3 kPa produces a distillate of 95 mol % acetone (A), 5 mol % water, and a residue containing
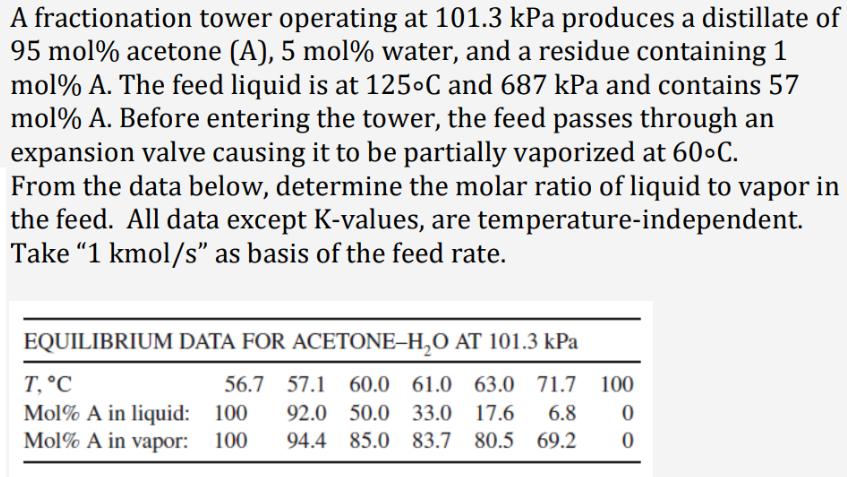
A fractionation tower operating at 101.3 kPa produces a distillate of 95 mol % acetone (A), 5 mol % water, and a residue containing 1 mol% A. The feed liquid is at 125C and 687 kPa and contains 57 mol % A. Before entering the tower, the feed passes through an expansion valve causing it to be partially vaporized at 60C. From the data below, determine the molar ratio of liquid to vapor in the feed. All data except K-values, are temperature-independent. Take "1 kmol/s" as basis of the feed rate. EQUILIBRIUM DATA FOR ACETONE-HO AT 101.3 kPa 56.7 57.1 60.0 61.0 63.0 71.7 100 92.0 50.0 33.0 17.6 6.8 0 94.4 85.0 83.7 80.5 69.2 0 T, C Mol% A in liquid: 100 Mol% A in vapor: 100
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Separation process principles
Authors: J. D. Seader
2nd Edition
471464805, 978-0471464808
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



