Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A fuel cell can operate at very high temperatures, meaning the heat produced can be used to provide additional power (Combined-Cycle, not CHP). If
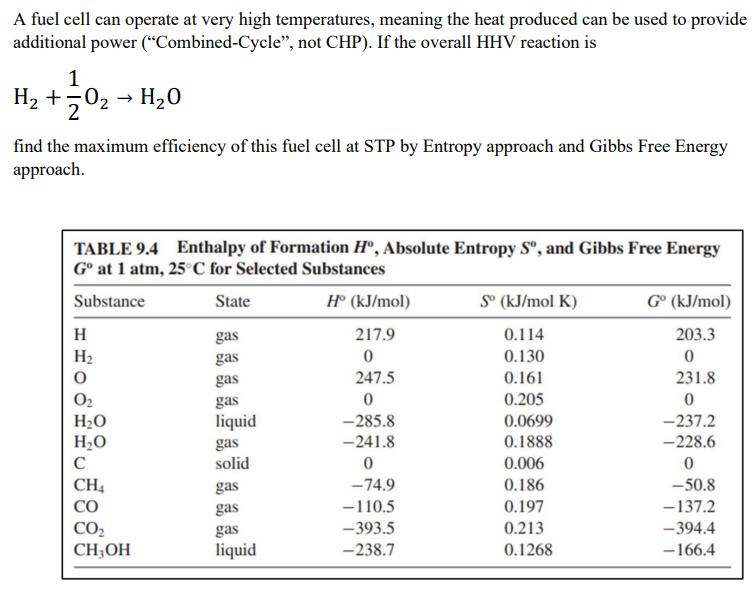
A fuel cell can operate at very high temperatures, meaning the heat produced can be used to provide additional power ("Combined-Cycle", not CHP). If the overall HHV reaction is 1 H +50 H0 2 find the maximum efficiency of this fuel cell at STP by Entropy approach and Gibbs Free Energy approach. TABLE 9.4 Enthalpy of Formation H, Absolute Entropy S, and Gibbs Free Energy G at 1 atm, 25C for Selected Substances Substance State gas gas H H O 0 HO HO C CH CO CO CHOH gas gas liquid gas solid gas gas gas liquid H (kJ/mol) 217.9 0 247.5 0 -285.8 -241.8 0 -74.9 -110.5 -393.5 -238.7 S (KJ/mol K) 0.114 0.130 0.161 0.205 0.0699 0.1888 0.006 0.186 0.197 0.213 0.1268 G (kJ/mol) 203.3 0 231.8 0 -237.2 -228.6 0 -50.8 -137.2 -394.4 -166.4
Step by Step Solution
★★★★★
3.50 Rating (150 Votes )
There are 3 Steps involved in it
Step: 1
Given the fuel cell reaction H2 12 O2 H2O We can calculate the maximum effici...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


