Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A fuel oil is analyzed and found to contain 83.86 wt% carbon, 13.40% elemental hydrogen (H),1.90% sulfur, and the remainder noncombustible matter. The oil is
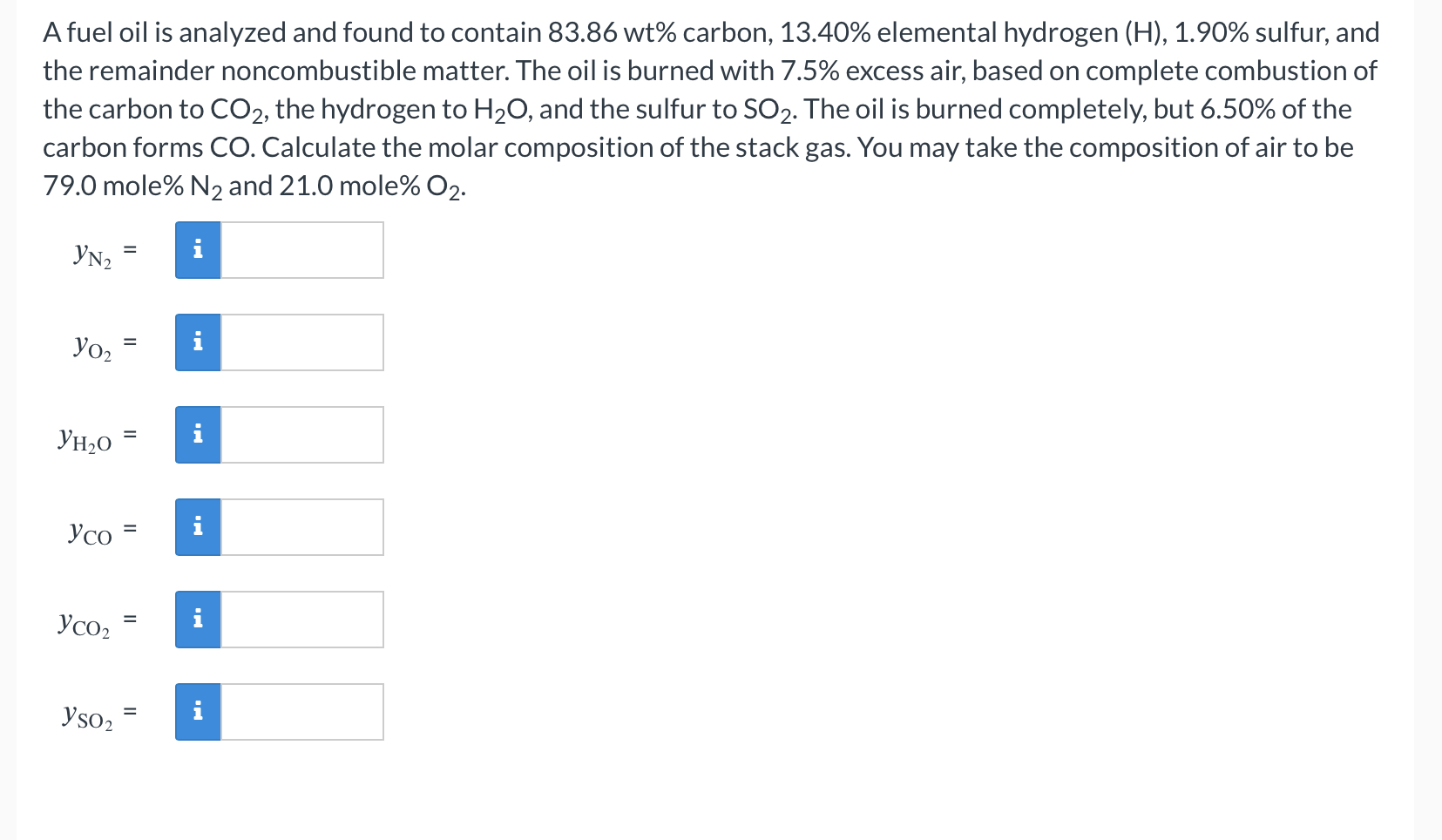 A fuel oil is analyzed and found to contain 83.86 wt\% carbon, 13.40% elemental hydrogen (H),1.90% sulfur, and the remainder noncombustible matter. The oil is burned with 7.5% excess air, based on complete combustion of the carbon to CO2, the hydrogen to H2O, and the sulfur to SO2. The oil is burned completely, but 6.50% of the carbon forms CO. Calculate the molar composition of the stack gas. You may take the composition of air to be 79.0 mole\% N2 and 21.0 mole\% O2. yN2= yO2= yH2O= yCO= yCO2= ySO2=
A fuel oil is analyzed and found to contain 83.86 wt\% carbon, 13.40% elemental hydrogen (H),1.90% sulfur, and the remainder noncombustible matter. The oil is burned with 7.5% excess air, based on complete combustion of the carbon to CO2, the hydrogen to H2O, and the sulfur to SO2. The oil is burned completely, but 6.50% of the carbon forms CO. Calculate the molar composition of the stack gas. You may take the composition of air to be 79.0 mole\% N2 and 21.0 mole\% O2. yN2= yO2= yH2O= yCO= yCO2= ySO2= Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


