Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A gas evolved during a chemical reaction and has a volume of 612 mL at 34 C and 1,191 torr. What was the volume
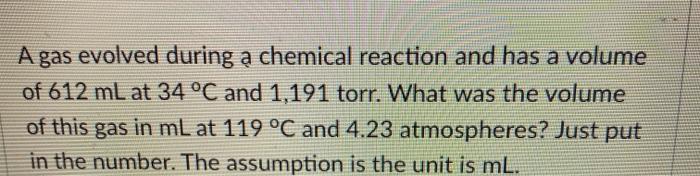

A gas evolved during a chemical reaction and has a volume of 612 mL at 34 C and 1,191 torr. What was the volume of this gas in mL at 119 C and 4.23 atmospheres? Just put in the number. The assumption is the unit is mL. A sample of aluminum metal reacts completely with an excess of hydrochloric acid: 2 Al(s) + 6 HCl(aq) --> 2AlCl3(aq) + 3 H(g) The hydrogen gas produced is collected over water at 28.0 C, so that there was a mixture of water vapor and hydrogen in the gas state. The water vapor was then removed with a drying agent so that the gas is pure hydrogen.The volume of the pure gas is 1,012 mL, and the total pressure in the gas collection vessel with just hydrogen, H gas is 113.2 kpa (kilopascal). Calculate the mass of aluminum metal that reacted in grams. Just put in the number. The assumption is the unit is grams. 1 atm = 760 torr = 760 mmHg = 101,325 pa = 101.325 kpa 1.01325 barr = 14.696 p.s.i. = 29.921 inHg = (inHg and p.s.i. are approximations of the metric pressures and are subject to significant figures)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


