Question
A gas expands from I to F in the figure below. The energy added to the gas by heat is 312 J when the
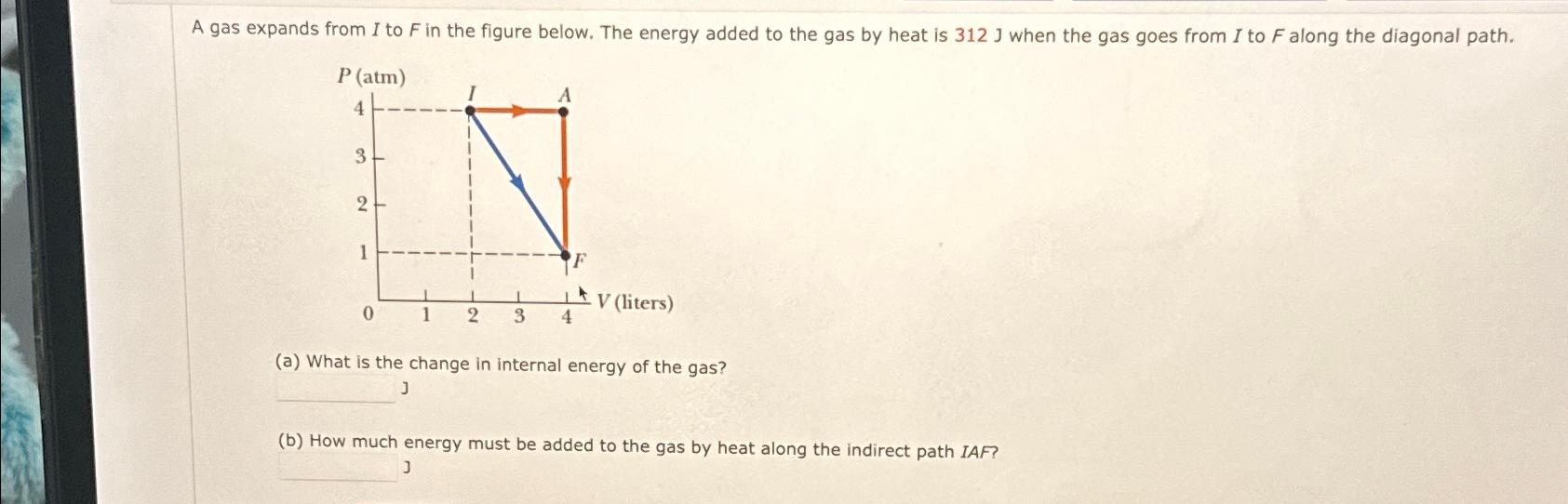
A gas expands from I to F in the figure below. The energy added to the gas by heat is 312 J when the gas goes from I to F along the diagonal path. P (atm) 4 3 2 1 0 1 3 4 AV (liters) (a) What is the change in internal energy of the gas? J (b) How much energy must be added to the gas by heat along the indirect path IAF? J
Step by Step Solution
There are 3 Steps involved in it
Step: 1
Analyzing the Gas Expansion We can use the first law of thermodynamics to analyze the gas expansion processThis law states that the total energy of a ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Physics
Authors: David Young, Shane Stadler
10th edition
1118486897, 978-1118836873, 1118836871, 978-1118899205, 1118899202, 978-1118486894
Students also viewed these Physics questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



