Question
A gas stream consisting of 65 mole% H2, 34.5 mole% CO, and 0.5 mole% CH4 is fed to a process to synthesize methanol (CO +
A gas stream consisting of 65 mole% H2, 34.5 mole% CO, and 0.5 mole% CH4 is fed to a process to synthesize methanol (CO + 2H2 ? CH3OH). The stream from the reactor is fed to a condenser where essentially all of the synthesized methanol is condensed and all of the unreacted gases are recycled back to the reactor. To avoid a buildup of methane, a purge stream is removed from the recycle. The overall conversion of CO is 85% and the methane mole fraction in the reactor inlet [n3/(n1+n2+n3)] is 0.03.
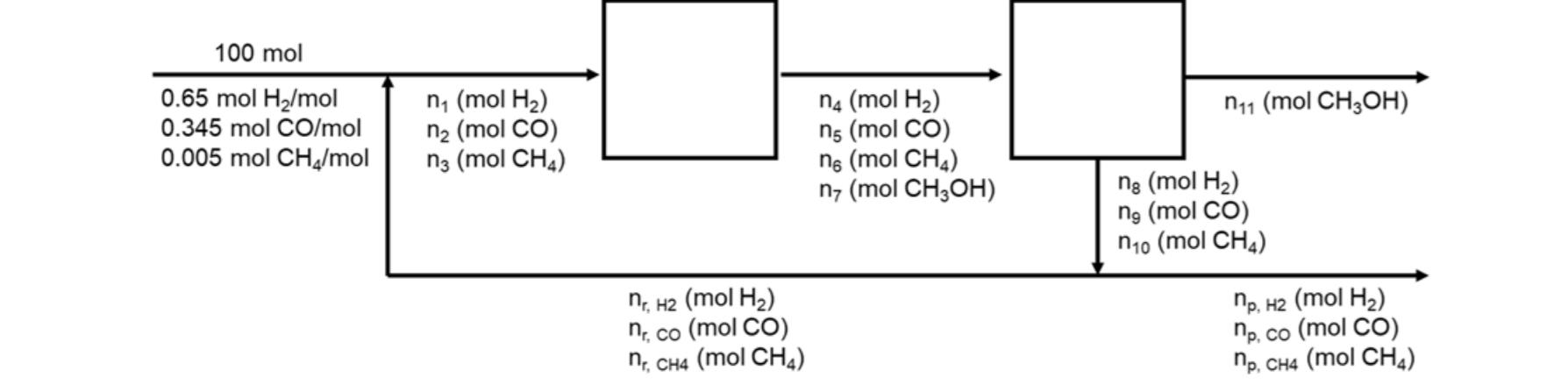
1. Find the amount of methanol produced by this process (n11).
2. Find the fraction of recycle that is purged [np/(nr+np)].
100 mol 0.65 mol H/mol 0.345 mol CO/mol 0.005 mol CH/mol n (mol H) n2 (mol CO) n3 (mol CH4) nr, H2 (mol H) nr, co (mol CO) nr, CH4 (mol CH4) n (mol H) ns (mol CO) n6 (mol CH) n7 (mol CH3OH) n1 (mol CH3OH) ng (mol H) ng (mol CO) n0 (mol CH4) np, H2 (mol H) np. co (mol CO) np, CH4 (mol CH4)
Step by Step Solution
3.49 Rating (166 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


