Answered step by step
Verified Expert Solution
Question
1 Approved Answer
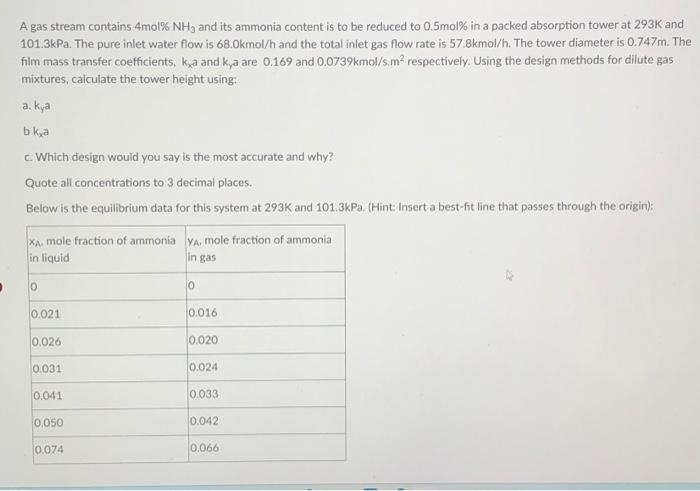
A gas stream contains 4mol % NH3 and its ammonia content is to be reduced to 0.5mol% in a packed absorption tower at 293K and
A gas stream contains 4mol % NH3 and its ammonia content is to be reduced to 0.5mol% in a packed absorption tower at 293K and 101.3kPa. The pure inlet water flow is 68.0kmol/h and the total inlet gas flow rate is 57.8kmol/h. The tower diameter is 0.747m. The film mass transfer coefficients, ka and kya are 0.169 and 0.0739kmol/s.m respectively. Using the design methods for dilute gas mixtures, calculate the tower height using: a. kya b kxa c. Which design would you say is the most accurate and why? Quote all concentrations to 3 decimal places. Below is the equilibrium data for this system at 293K and 101.3kPa. (Hint: Insert a best-fit line that passes through the origin): XA, mole fraction of ammonia YA, mole fraction of ammonia in gas in liquid 0 0.021 0.026 0.031 0.041 0.050 0.074 0 0.016 0.020 0.024 0.033 0.042 0.066
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


