Question
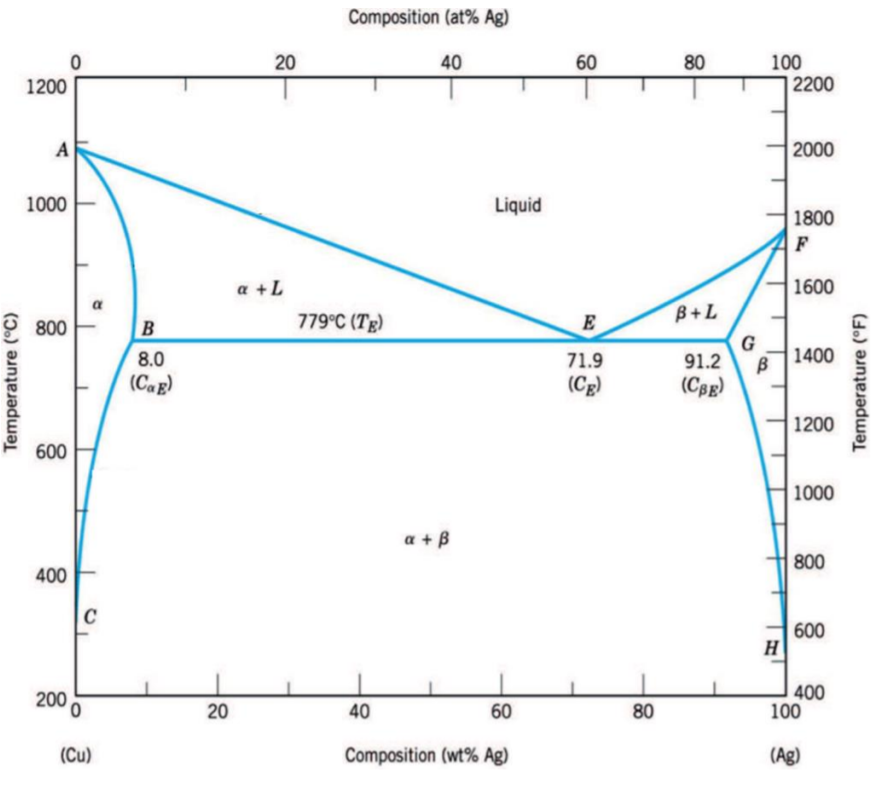
a- Give the melting temperature of the silver and the eutectic alloy in degrees Celsius; b- Give the solubility of Cu atoms in solid Ag
a- Give the melting temperature of the silver and the eutectic alloy in degrees Celsius; b- Give the solubility of Cu atoms in solid Ag solution in atomic percentage at 800C. Consider an alloy with 10% by weight copper (Cu) and 90% silver (Ag) and determine: c- the composition of the first solid to be formed during solidification; d- the composition of the last drop of liquid during solidification; e- the composition of the most Ag-rich phase at 850C; f- the mass fractions of the phases present at 600C. g- the constituents forming the microstructure of the material at room temperature. h- draw the temperature-time cooling curve of the material.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


