Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A handbook lists the density of lead as 11.3 g/mL. Several groups of students are attempting to determine the density of a lead weight
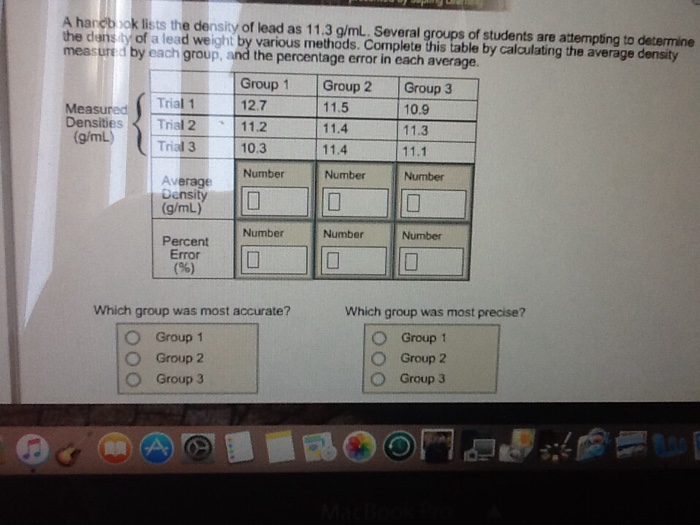
A handbook lists the density of lead as 11.3 g/mL. Several groups of students are attempting to determine the density of a lead weight by various methods. Complete this table by calculating the average density measured by each group, and the percentage error in each average. Measured Densities (g/mL) Trial 1 Trial 2 Trial 3 Average Density (g/mL) Percent Error (%) A Group 1 12.7 11.2 10.3 Number Which group was most accurate? Group 1 Group 2 Group 3 **** Number 0 Group 2 11.5 11.4 11.4 Number Number 0 Group 3 10.9 11.3 11.1 Number 0 Number 0 Which group was most precise? Group 1 Group 2 Group 3
Step by Step Solution
★★★★★
3.39 Rating (152 Votes )
There are 3 Steps involved in it
Step: 1
Group 1 Calculate the average density gmL as follows Density Trial 1Trial 2 Trial 3 Avera...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


