Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A healthcare company decides to market a hand warmer. It is basi- cally a palm-size plastic pouch containing a supersaturated aqueous salt solution; that
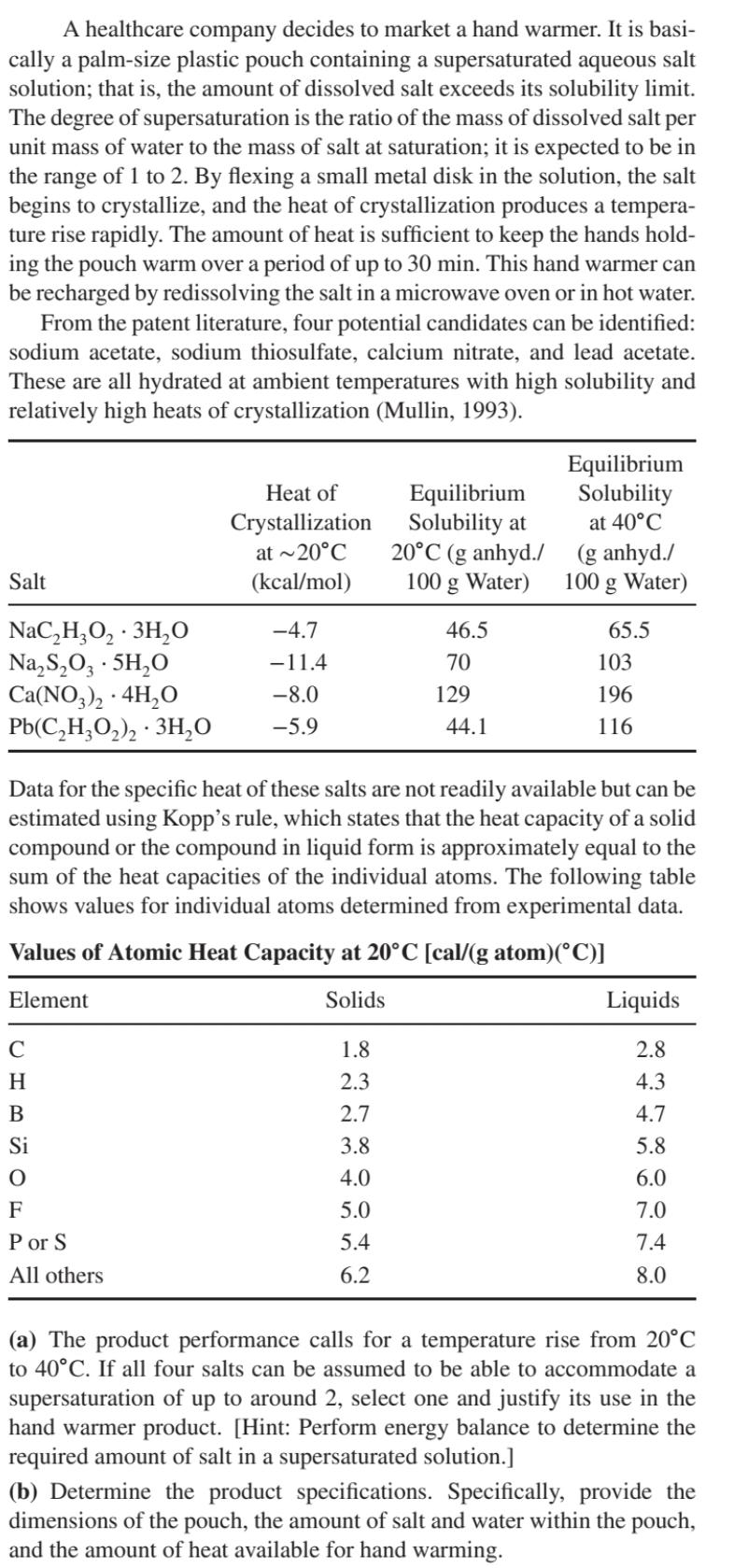
A healthcare company decides to market a hand warmer. It is basi- cally a palm-size plastic pouch containing a supersaturated aqueous salt solution; that is, the amount of dissolved salt exceeds its solubility limit. The degree of supersaturation is the ratio of the mass of dissolved salt per unit mass of water to the mass of salt at saturation; it is expected to be in the range of 1 to 2. By flexing a small metal disk in the solution, the salt begins to crystallize, and the heat of crystallization produces a tempera- ture rise rapidly. The amount of heat is sufficient to keep the hands hold- ing the pouch warm over a period of up to 30 min. This hand warmer can be recharged by redissolving the salt in a microwave oven or in hot water. From the patent literature, four potential candidates can be identified: sodium acetate, sodium thiosulfate, calcium nitrate, and lead acetate. These are all hydrated at ambient temperatures with high solubility and relatively high heats of crystallization (Mullin, 1993). Heat of Crystallization Equilibrium Equilibrium Solubility Solubility at at 40C Salt at ~20C (kcal/mol) 20C (g anhyd./ (g anhyd./ 100 g Water) 100 g Water) NaCHO 3HO NaSO, 5HO -4.7 46.5 65.5 -11.4 70 103 Ca(NO3)2 + 4HO . -8.0 129 196 Pb(C2H3O2)2.3HO -5.9 44.1 116 Data for the specific heat of these salts are not readily available but can be estimated using Kopp's rule, which states that the heat capacity of a solid compound or the compound in liquid form is approximately equal to the sum of the heat capacities of the individual atoms. The following table shows values for individual atoms determined from experimental data. Values of Atomic Heat Capacity at 20C [cal/(g atom)(C)] Element C Solids Liquids 1.8 2.8 H 2.3 4.3 B 2.7 4.7 Si 3.8 5.8 4.0 6.0 F Por S All others 5.0 7.0 5.4 7.4 6.2 8.0 (a) The product performance calls for a temperature rise from 20C to 40C. If all four salts can be assumed to be able to accommodate a supersaturation of up to around 2, select one and justify its use in the hand warmer product. [Hint: Perform energy balance to determine the required amount of salt in a supersaturated solution.] (b) Determine the product specifications. Specifically, provide the dimensions of the pouch, the amount of salt and water within the pouch, and the amount of heat available for hand warming.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


