Question
A homogeneous reaction in which reactant A is converted into product D occurs in a spherical gel in which catalyst components are uniformly dispersed, and
A homogeneous reaction in which reactant A is converted into product D occurs in a spherical gel in which catalyst components are uniformly dispersed, and in this case, the reaction formula may be represented as -k1cA. In addition, spherical gel is dispersed in water containing a certain concentration of A component (cA). (1) Name three valid assumptions about the above system. Establish a mass balance expression in the control volume inside the gel, and derive the final differential equation expressed for the concentration cA of the A component. (2) Refer to all boundary conditions for solving the differential equation obtained above. (3) Solve the differential equation above to explain how you can find the amount of A component consumed for one hour in a spherical gel when you obtain an analytical solution that represents the concentration distribution of A component as follows. (You just need to explain how to solve the problem without solving it)
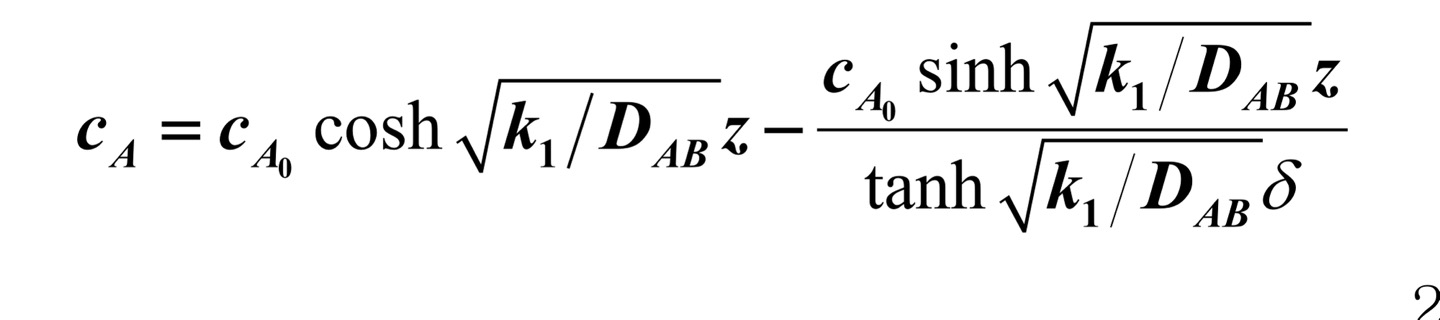 cA=cA0coshk1/DABztanhk1/DABcA0sinhk1/DABz cA=cA0coshk1/DABztanhk1/DABcA0sinhk1/DABz
cA=cA0coshk1/DABztanhk1/DABcA0sinhk1/DABz cA=cA0coshk1/DABztanhk1/DABcA0sinhk1/DABz Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


