Answered step by step
Verified Expert Solution
Question
1 Approved Answer
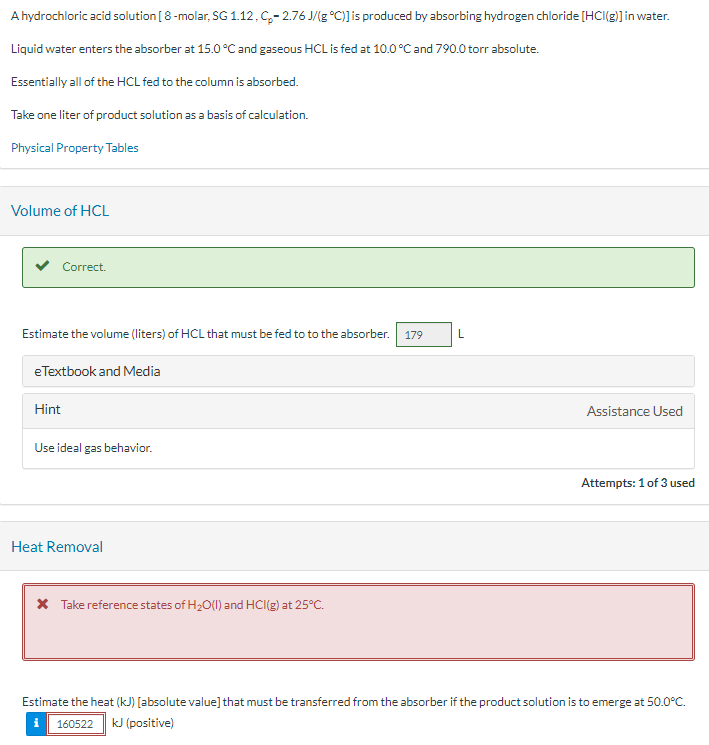
A hydrochloric acid solution [ 8 - molar, SG 1 . 1 2 , Cp = 2 . 7 6 J / ( g
A hydrochloric acid solution molar, SG Cp Jg deg C is produced by absorbing hydrogen chloride HClg in water. Liquid water enters the absorber at deg C and gaseous HCL is fed at deg C and torr absolute Essentially all of the HCL fed to the column is absorbed Take one liter of product solution as a basis of calculation. Estimate the volume liters of HCL that must be fed to to the absorber L Estimate the heat kJ that must be transferred from the absorber if the product solution is to emerge at deg C kJ Estimate the final solution temperature if the absorber operates adiabatically if it did not boil first deg CA hydrochloric acid solution molar, is produced by absorbing hydrogen chloride in water.
Liquid water enters the absorber at and gaseous is fed at and torr absolute
Essentially all of the fed to the column is absorbed
Take one liter of product solution as a basis of calculation.
Physical Property Tables
Volume of HCL
Correct.
Estimate the volume liters of HCL that must be fed to to the absorber
eTextbook and Media
Hint
Heat Removal
Take reference states of and at
Estimate the heat absolute value that must be transferred from the absorber if the product solution is to emerge at
please help with part b
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


