Question: A piston-cylinder device contains air which can be assumed as an ideal gas. Initially, the specific entropy of the air is 2.0 kJ/kgK and
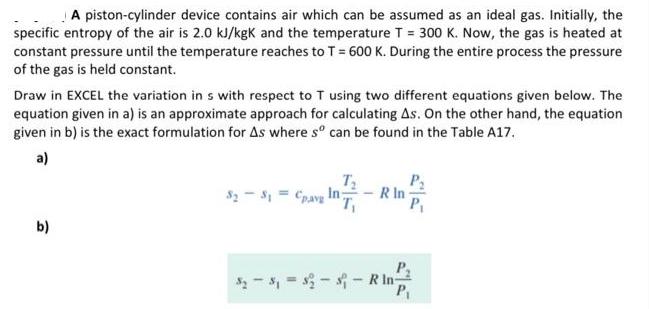
A piston-cylinder device contains air which can be assumed as an ideal gas. Initially, the specific entropy of the air is 2.0 kJ/kgK and the temperature T = 300 K. Now, the gas is heated at constant pressure until the temperature reaches to T = 600 K. During the entire process the pressure of the gas is held constant. Draw in EXCEL the variation in s with respect to T using two different equations given below. The equation given in a) is an approximate approach for calculating As. On the other hand, the equation given in b) is the exact formulation for As where so can be found in the Table A17. a) b) T $23, Cpavy In; R In P ---R In P
Step by Step Solution
3.46 Rating (156 Votes )
There are 3 Steps involved in it
a Approximate Approach Equation for As The approximate equation for calculating specific entropy change s is s Cp lnT2T1 In this equation Cp represent... View full answer

Get step-by-step solutions from verified subject matter experts


