Question
(a) In a recent synthesis of the cyclic peptide 13, which has antibiotic activity, the authors chose the disconnection point illustrated in the retrosynthetic scheme
(a) In a recent synthesis of the cyclic peptide 13, which has antibiotic activity, the authors chose the disconnection point illustrated in the retrosynthetic scheme below, to give two protected fragments 14 and 15.
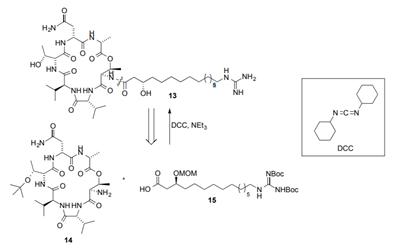
I. Suggest a reason why this might be considered a good point for a first disconnection. 1 mark
II. Fragments 14 and 15 could be connected through an amide bond formation using DCC and a base (NEt3) as the reagents. Provide a mechanism for this reaction. (Hint: Use the general structures R1 -NH2 and R2 -CO2H to save drawing out the full structures.) 5 marks
b) The synthesis of fragment 15 is given in the scheme below.
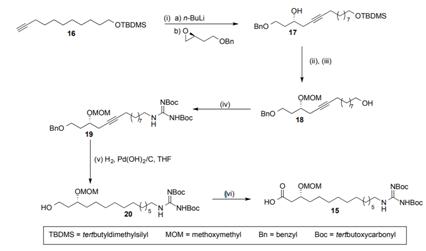
i. Suggest reagents that would achieve steps (ii) and (iii) and explain the order in which you choose to perform these steps. 3 marks
ii. Consider step (v) of the synthesis. Briefly explain the two transformations that occur in this step, then give two reasons for the choice of a benzyl ether to protect the alcohol of starting material
HN H HN NH2 NH HN OH NH 13 N=C=N DCC, NEI, DCC HN OMOM NBoc NH2 HO 15 NH HN 14
Step by Step Solution
3.38 Rating (164 Votes )
There are 3 Steps involved in it
Step: 1
Ans a Ans b i Deprotection of Methoxymethyl acetal MOM is generally done under acidic conditions whe...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


