Question
A. Is the reaction first order or second order with respect to [C12H22O11]? B. What is the rate constant? C. Using this rate constant, calculate
A. Is the reaction first order or second order with respect to [C12H22O11]?
B. What is the rate constant?
C. Using this rate constant, calculate the concentration of sucrose at 39, 80, 140, and 210 min if the initial sucrose concentration was 0.316 M and the reaction was first order in sucrose. Express your answers using two significant figures separated by commas.
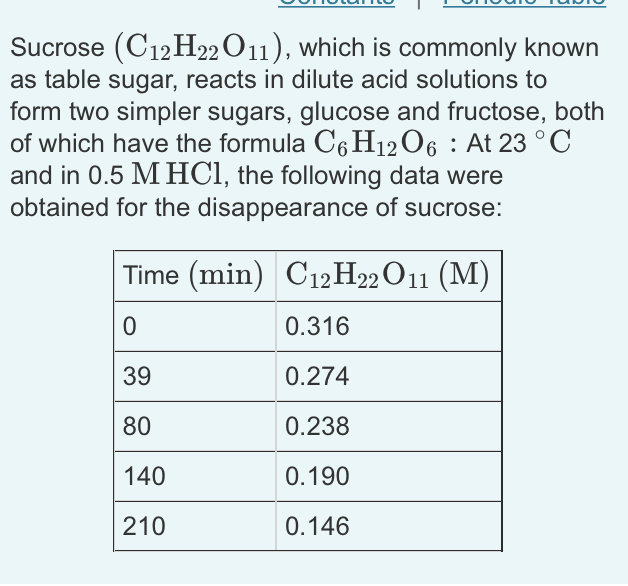
Sucrose (C12H22 O11), which is commonly known as table sugar, reacts in dilute acid solutions to form two simpler sugars, glucose and fructose, both of which have the formula C6H12O6 : At 23 C and in 0.5 M HCl, the following data were obtained for the disappearance of sucrose: Time (min) C12H22 O11 (M) 0 0.316 39 0.274 80 0.238 140 0.190 210 0.146
Step by Step Solution
3.45 Rating (152 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Chemistry The Central Science
Authors: Theodore Brown, Eugene LeMay, Bruce Bursten, Catherine Murphy, Patrick Woodward
12th edition
321696727, 978-0132175081, 978-0321696724
Students also viewed these Accounting questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



