Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A liquid mixture of A and B displays an azeotrope at A = 0.7 at 384 K and a pressure of 3.500 bar. The
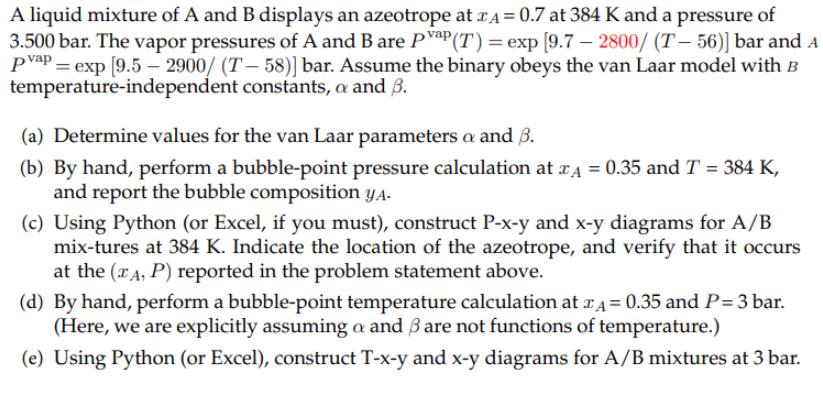
A liquid mixture of A and B displays an azeotrope at A = 0.7 at 384 K and a pressure of 3.500 bar. The vapor pressures of A and B are PP (T) = exp [9.7 - 2800/ (T-56)] bar and A pvap = exp [9.5-2900/ (T-58)] bar. Assume the binary obeys the van Laar model with B temperature-independent constants, a and 3. (a) Determine values for the van Laar parameters a and 3. (b) by hand, perform a bubble-point pressure calculation at x = 0.35 and T = 384 K, and report the bubble composition y. (c) Using Python (or Excel, if you must), construct P-x-y and x-y diagrams for A/B mix-tures at 384 K. Indicate the location of the azeotrope, and verify that it occurs at the (A,P) reported in the problem statement above. (d) By hand, perform a bubble-point temperature calculation at = 0.35 and P= 3 bar. (Here, we are explicitly assuming a and 3 are not functions of temperature.) (e) Using Python (or Excel), construct T-x-y and x-y diagrams for A/B mixtures at 3 bar.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


