Answered step by step
Verified Expert Solution
Question
1 Approved Answer
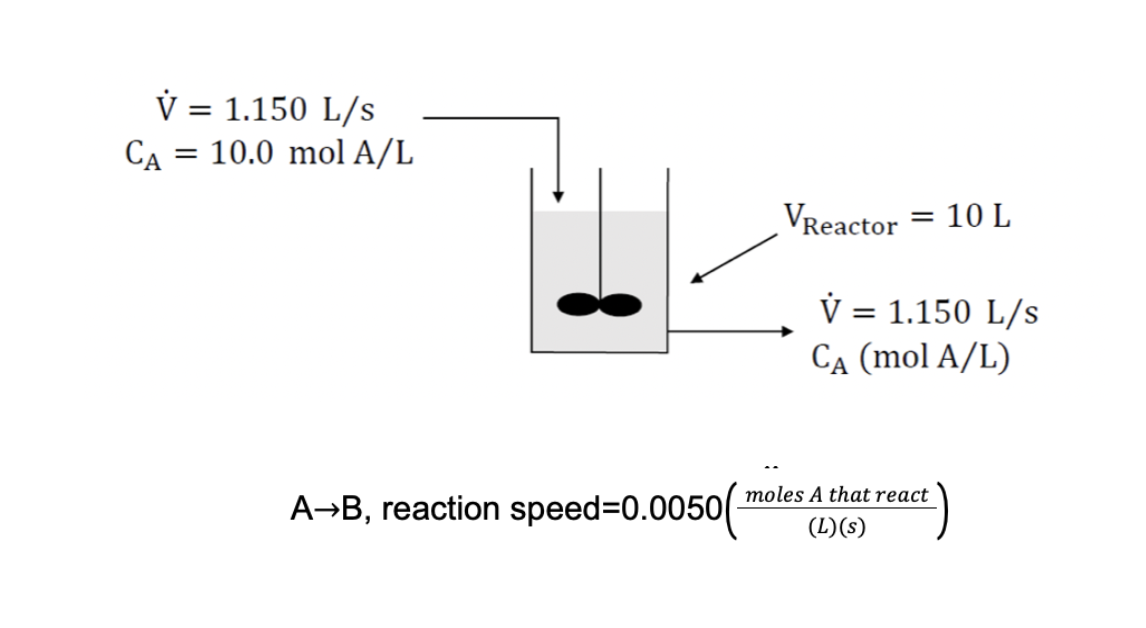
A liquid phase reaction with AB stoichiometry is carried out in a 1 0 . 0 liter, well - mixed, continuous stirred tank reactor, a
A liquid phase reaction with AB stoichiometry is carried out in a liter, wellmixed, continuous stirred tank reactor, a schematic of the process is shown below: continuous stirred tank reactor, a schematic of the process is shown below:
It can be considered that the reactor mixture is perfect, so that the content is uniform and the concentration of A in the product stream is equal to that inside the tank. Initially the tank is filled with a solution containing mol AL and then the inlet and outlet flows are initiated.
Write the molar balance for species A and calculateCAS, that is steady state concentration.
Obtain the expression CAt
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


