Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The elementary, irreversible gas-phase reaction A+B--> 2 P is carried out non-isothermally in a PFR that has no heat exchanger around it. An equimolar
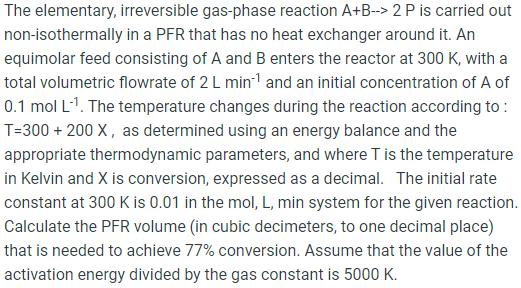
The elementary, irreversible gas-phase reaction A+B--> 2 P is carried out non-isothermally in a PFR that has no heat exchanger around it. An equimolar feed consisting of A and B enters the reactor at 300 K, with a total volumetric flowrate of 2 L min1 and an initial concentration of A of 0.1 mol L-1. The temperature changes during the reaction according to: T=300 + 200 X, as determined using an energy balance and the appropriate thermodynamic parameters, and where T is the temperature in Kelvin and X is conversion, expressed as a decimal. The initial rate constant at 300 K is 0.01 in the mol, L, min system for the given reaction. Calculate the PFR volume (in cubic decimeters, to one decimal place) that is needed to achieve 77% conversion. Assume that the value of the activation energy divided by the gas constant is 5000 K.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


