Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A mechanical vapour compression refrigerator that uses R-22 as working refrigerant is referred as in Figure 1. Assume the ambient temperature (T.) and the
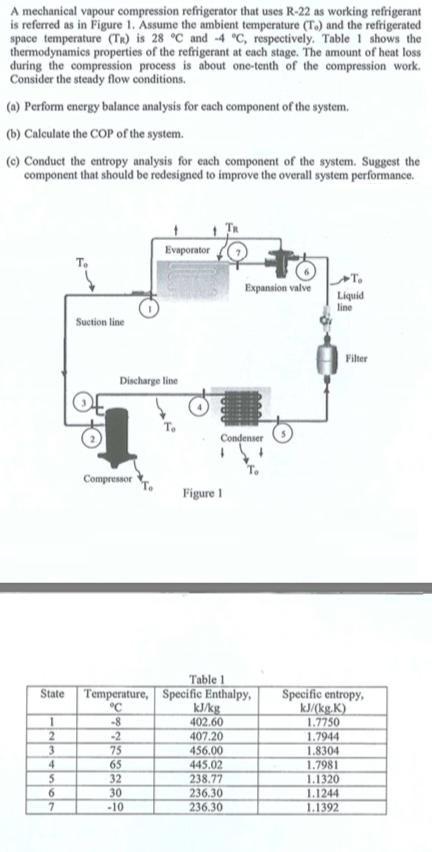
A mechanical vapour compression refrigerator that uses R-22 as working refrigerant is referred as in Figure 1. Assume the ambient temperature (T.) and the refrigerated space temperature (TR) is 28 C and -4 C, respectively. Table 1 shows the thermodynamics properties of the refrigerant at each stage. The amount of heat loss during the compression process is about one-tenth of the compression work. Consider the steady flow conditions. (a) Perform energy balance analysis for each component of the system. (b) Calculate the COP of the system. (c) Conduct the entropy analysis for each component of the system. Suggest the component that should be redesigned to improve the overall system performance. State 1 2 3 4 5 6 7 Suction line Discharge line Compressor Evaporator 75 65 32 30 -10 Condenser Figure 1 Expansion valve Table 1 Temperature, Specific Enthalpy, -8 kJ/kg 402.60 407.20 456.00 445.02 238.77 236.30 236.30 To Liquid line Filter Specific entropy, kJ/(kg.K) 1.7750 1.7944 1.8304 1.7981 1.1320 1.1244 1.1392
Step by Step Solution
★★★★★
3.39 Rating (149 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


