Answered step by step
Verified Expert Solution
Question
1 Approved Answer
?? A medical technician is working with the four samples of radionuclides listed in the table below. Initially, each sample contains 5.00 mol of the
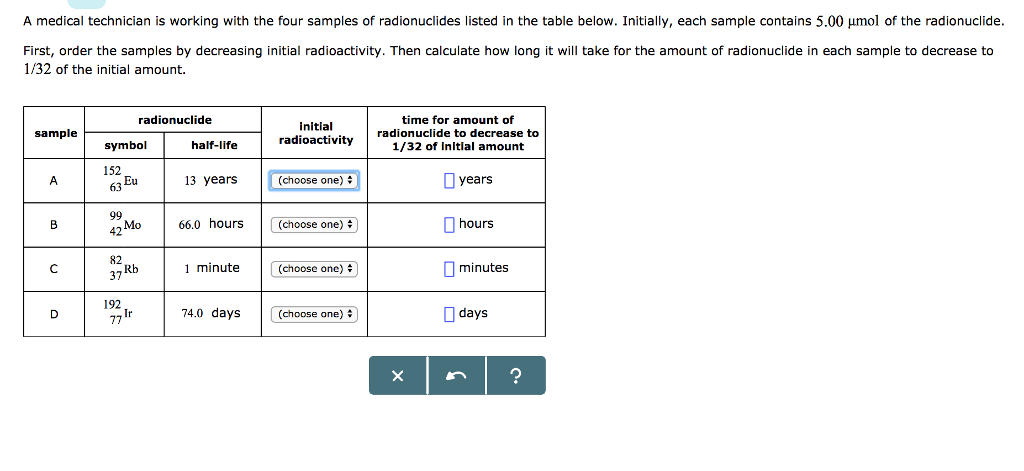
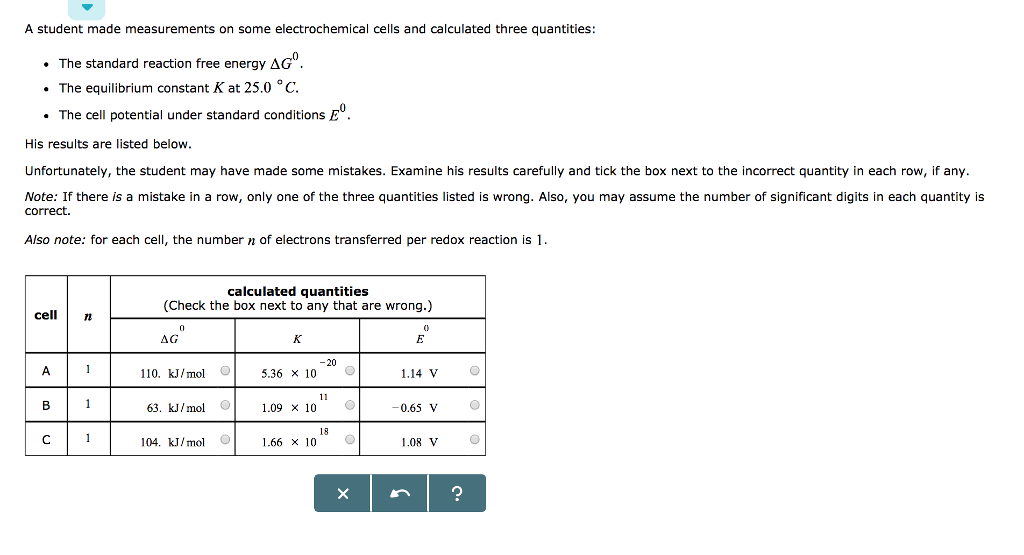
A medical technician is working with the four samples of radionuclides listed in the table below. Initially, each sample contains 5.00 mol of the radionuclide. First, order the samples by decreasing initial radioactivity. Then calculate how long will take for the amount of radionuclide in each sample to decrease to 1/32 of the initial amount. sample A B D symbol 152 63 Eu 99 42 Mo 82 37 Rb radionuclide 192 77 Ir half-life 13 years. 66.0 hours 1 minute 74.0 days initial radioactivity (choose one) (choose one) + (choose one) # (choose one) time for amount of radionuclide to decrease to 1/32 of Initial amount X years hours minutes days ? A student made measurements on some electrochemical cells and calculated three quantities: The standard reaction free energy AG. The equilibrium constant K at 25.0 C. The cell potential under standard conditions His results are listed below. Unfortunately, the student may have made some mistakes. Examine his results carefully and tick the box next to the incorrect quantity in each row, if any. Note: If there is a mistake in a row, only one of the three quantities listed is wrong. Also, you may assume the number of significant digits in each quantity is correct. Also note: for each cell, the number of electrons transferred per redox reaction is 1. cell 72 A B 1 1 1 calculated quantities (Check the box next to any that are wrong.) AG 0 110. kJ/mol O 63. kJ/mol 104. kJ/mol K -20 5.36 x 10 C 1.09 x 10 11 18 1.66 x 10. O O X E 1.14 V -0.65 V 1.08 V ? O O O
Step by Step Solution
★★★★★
3.62 Rating (167 Votes )
There are 3 Steps involved in it
Step: 1
Lower the half life higher will be initial radioactivity so initial radio activity Samp...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


