Question
A mercury manometer is used to measure the amount of gas confined in a tube if the mercury in the branch connected to the
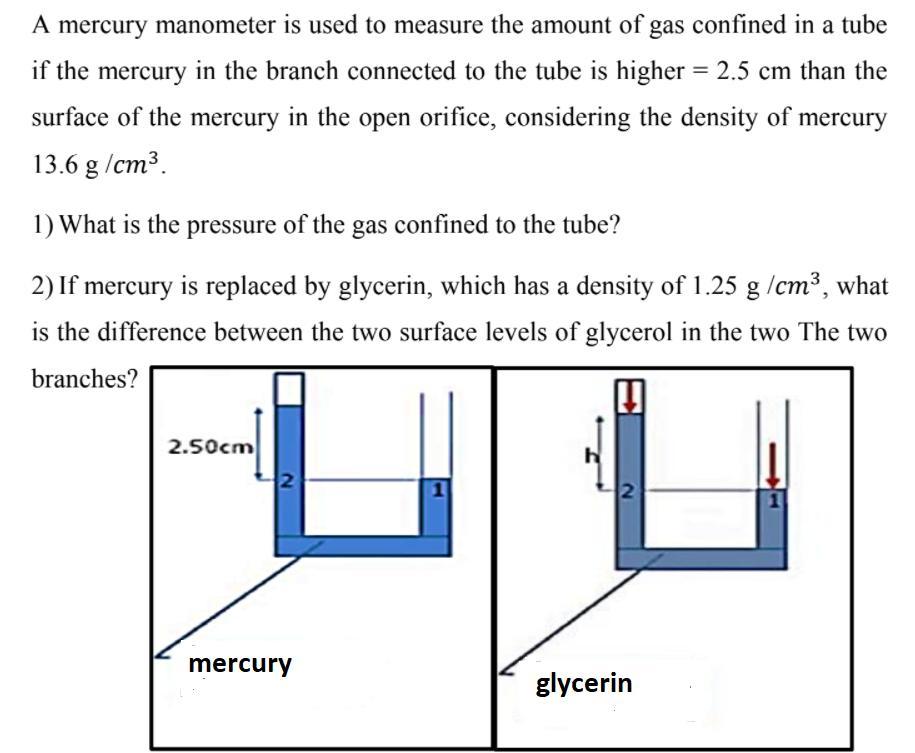
A mercury manometer is used to measure the amount of gas confined in a tube if the mercury in the branch connected to the tube is higher = 2.5 cm than the surface of the mercury in the open orifice, considering the density of mercury 13.6 g /cm3. 1) What is the pressure of the gas confined to the tube? 2) If mercury is replaced by glycerin, which has a density of 1.25 g /cm?, what is the difference between the two surface levels of glycerol in the two The two branches? 2.50cm mercury glycerin
Step by Step Solution
3.41 Rating (164 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Fundamentals of Aerodynamics
Authors: J. Anderson
2nd Edition
978-0071254083, 1259129918, 71254080, 70016798, 9781259129919, 978-0070016798
Students also viewed these Mechanical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



