Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A mixture of gaseous reactants is put into a cylinder, where a chemical reaction turns them into gaseous products. The cylinder has a piston that
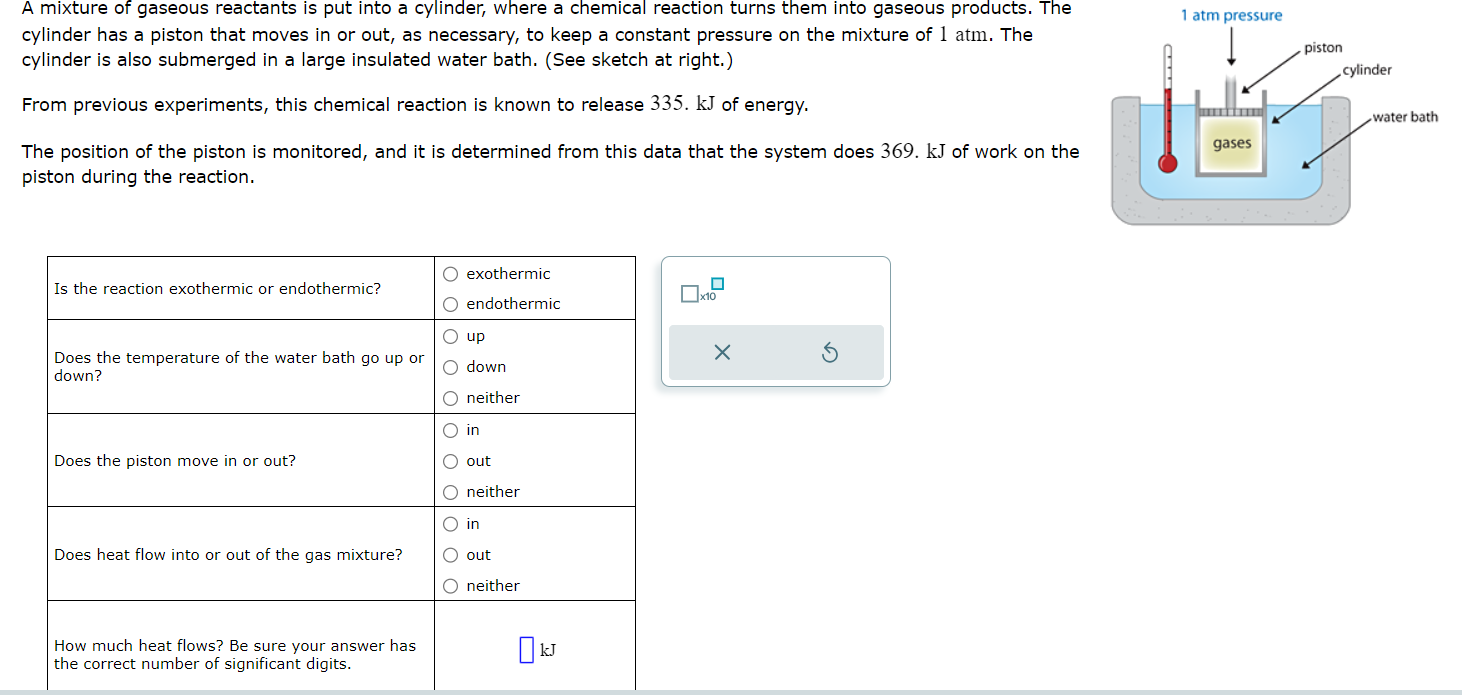
A mixture of gaseous reactants is put into a cylinder, where a chemical reaction turns them into gaseous products. The
cylinder has a piston that moves in or out, as necessary, to keep a constant pressure on the mixture of atm. The
cylinder is also submerged in a large insulated water bath. See sketch at right.
From previous experiments, this chemical reaction is known to release of energy.
The position of the piston is monitored, and it is determined from this data that the system does of work on the
piston during the reaction.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


