Question
A mixture of ideal gas contains 5 moles of monatomic gas and 1 mole of rigid diatomic gas is initially at Vo pressure Po,
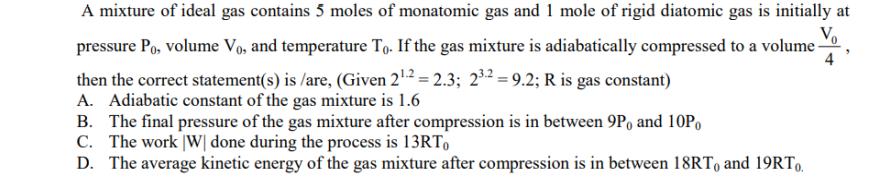
A mixture of ideal gas contains 5 moles of monatomic gas and 1 mole of rigid diatomic gas is initially at Vo pressure Po, volume Vo, and temperature To. If the gas mixture is adiabatically compressed to a volume- then the correct statement(s) is/are, (Given 2.2 = 2.3; 23.2=9.2; R is gas constant) 4 A. Adiabatic constant of the gas mixture is 1.6 B. The final pressure of the gas mixture after compression is in between 9P, and 10Po C. The work W done during the process is 13RT D. The average kinetic energy of the gas mixture after compression is in between 18RT and 19RTO.
Step by Step Solution
3.45 Rating (152 Votes )
There are 3 Steps involved in it
Step: 1
The detailed ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Thermodynamics An Engineering Approach
Authors: Yunus A. Cengel, Michael A. Boles
8th edition
73398179, 978-0073398174
Students also viewed these Physics questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



