Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A mixture of O2, CO, and He gases with mass fraction of 0.0625, 0.625, and 0.3125 respectively enter an adiabatic turbine at 1000 kPa
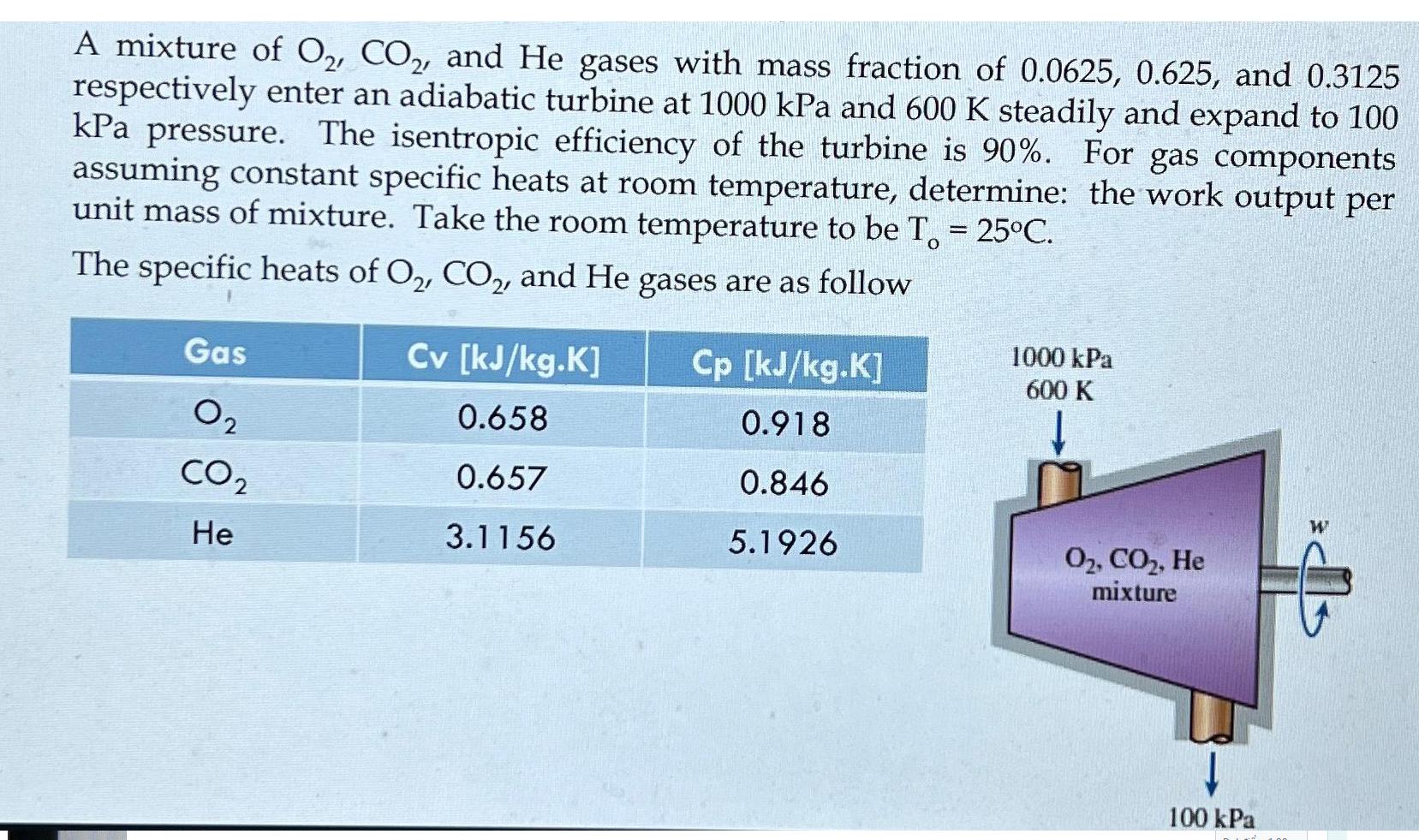
A mixture of O2, CO, and He gases with mass fraction of 0.0625, 0.625, and 0.3125 respectively enter an adiabatic turbine at 1000 kPa and 600 K steadily and expand to 100 kPa pressure. The isentropic efficiency of the turbine is 90%. For gas components assuming constant specific heats at room temperature, determine: the work output per unit mass of mixture. Take the room temperature to be To = 25C. The specific heats of O, CO, and He gases are as follow Gas 0 CO He Cv [kJ/kg.K] 0.658 0.657 3.1156 Cp [kJ/kg.K] 0.918 0.846 5.1926 1000 kPa 600 K O2, CO, He mixture 100 kPa W
Step by Step Solution
There are 3 Steps involved in it
Step: 1
To find the work output per unit mass of the mixture exiting the adiabatic turbine well need to use the principles of thermodynamics for mixtures and ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


