Question
A molecule of oxygen gas has chemical formula 02. If the temperature of oxygen gas in a container is 31C, find the root-mean-square speed
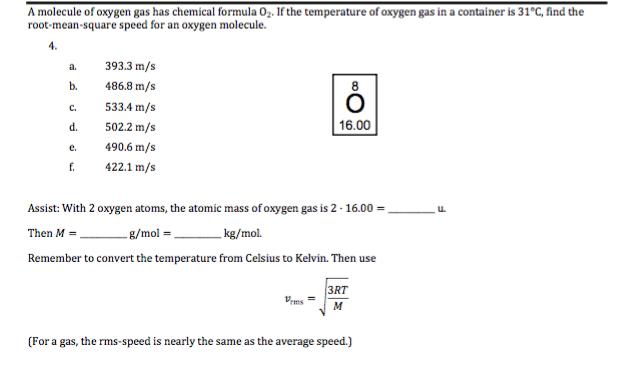
A molecule of oxygen gas has chemical formula 02. If the temperature of oxygen gas in a container is 31C, find the root-mean-square speed for an oxygen molecule. 4. a. 393.3 m/s b. 486.8 m/s C. 533.4 m/s d. 502.2 m/s e. 490.6 m/s f. 422.1 m/s 16.00 Assist: With 2 oxygen atoms, the atomic mass of oxygen gas is 2 - 16.00 Then M = g/mol = kg/mol. Remember to convert the temperature from Celsius to Kelvin. Then use 3RT M (For a gas, the rms-speed is nearly the same as the average speed.)
Step by Step Solution
3.44 Rating (154 Votes )
There are 3 Steps involved in it
Step: 1
Given information Molecule of Oxygen Gas O2 Chemical formula O2 Temperature 31 Degrees Celsius We wa...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Elementary Surveying An Introduction to Geomatics
Authors: Charles D. Ghilani, Paul R. Wolf
13th Edition
978-0132554343, 132554348, 978-0273751441
Students also viewed these Physics questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



