Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A newly developed synthetic affinity ligand for antibody adsorption is being both ligands are immobilised to the same chromatography beads. The synthetic matrix has shown
A newly developed synthetic affinity ligand for antibody adsorption is being
both ligands are immobilised to the same chromatography beads. The
synthetic matrix has shown comparable binding capacity when studied using
batch equilibrium experiments in the laboratory. The two matrices were then
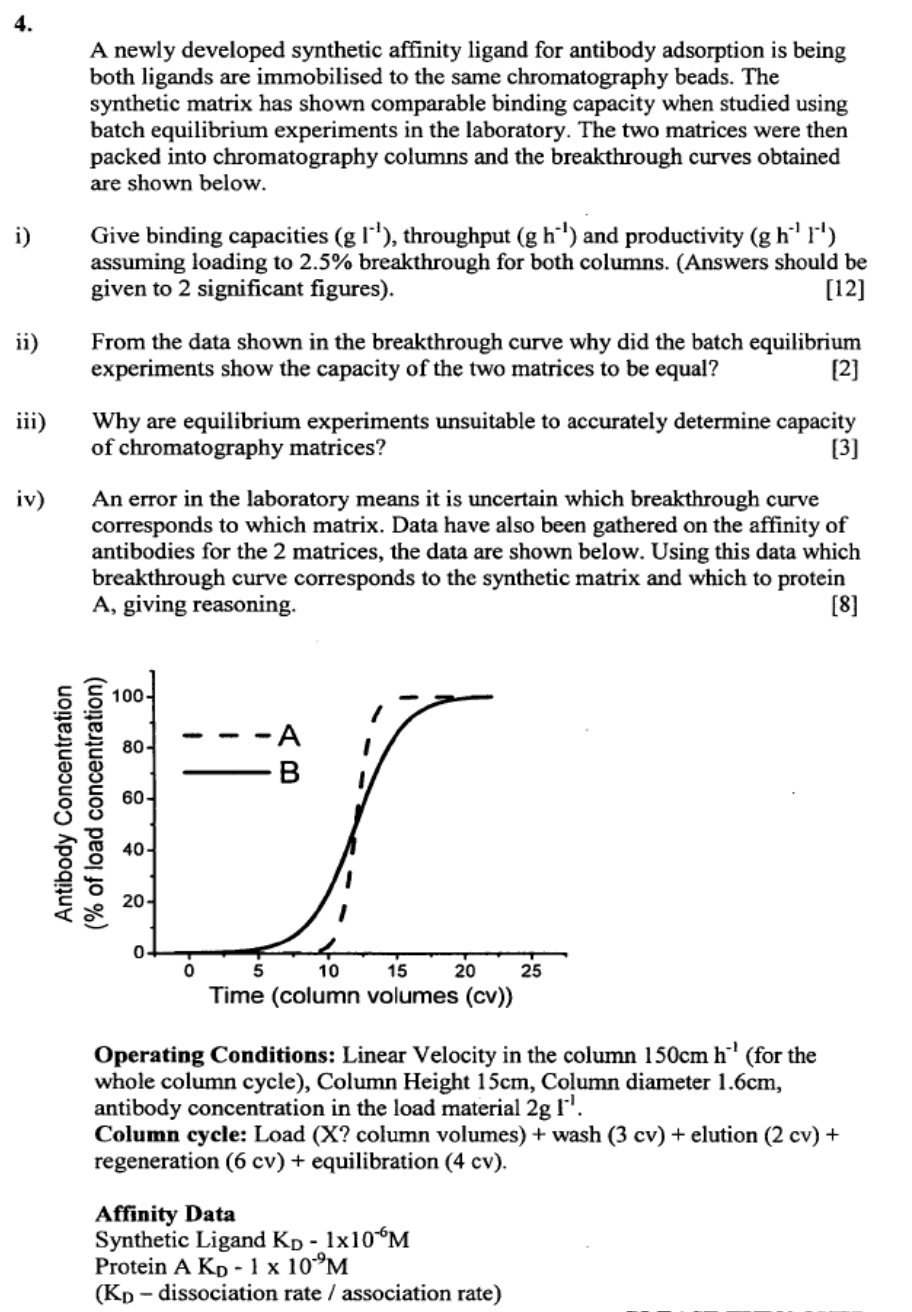
packed into chromatography columns and the breakthrough curves obtained
are shown below.
i Give binding capacities throughput and productivity
assuming loading to breakthrough for both columns. Answers should be
given to significant figures
ii From the data shown in the breakthrough curve why did the batch equilibrium
experiments show the capacity of the two matrices to be equal?
iii Why are equilibrium experiments unsuitable to accurately determine capacity
of chromatography matrices?
iv An error in the laboratory means it is uncertain which breakthrough curve
corresponds to which matrix. Data have also been gathered on the affinity of
antibodies for the matrices, the data are shown below. Using this data which
breakthrough curve corresponds to the synthetic matrix and which to protein
A giving reasoning.
Operating Conditions: Linear Velocity in the column for the
whole column cycle Column Height Column diameter
antibody concentration in the load material
Column cycle: Load X column volumes wash cv elution
regeneration cv equilibration
Affinity Data
Synthetic Ligand
Protein
dissociation rate association rate
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


