Answered step by step
Verified Expert Solution
Question
1 Approved Answer
TABLE 28.1 Typical cathode efficiencies in electroplating and values of plating constant C. Plate Metal Cadmium (2) Chromium (3) Copper (1) Gold (1) Nickel
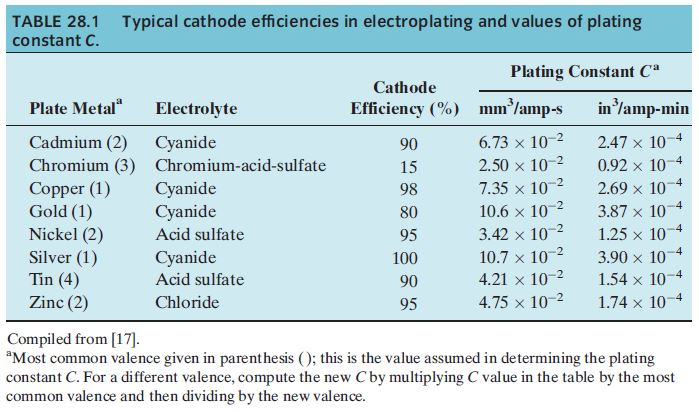
TABLE 28.1 Typical cathode efficiencies in electroplating and values of plating constant C. Plate Metal" Cadmium (2) Chromium (3) Copper (1) Gold (1) Nickel (2) Silver (1) Tin (4) Zinc (2) Electrolyte Cyanide Chromium-acid-sulfate Cyanide Cyanide Acid sulfate Cyanide Acid sulfate Chloride Cathode Efficiency (%) 90 15 98 80 95 100 90 95 Plating Constant Ca mm/amp-s -2 6.73 x 10-2 2.50 x 10- 7.35 x 10-2 10.6 x 10-2 3.42 x 10- 10.7 x 10-2 4.21 x 10- 4.75 x 10- in /amp-min -4 2.47 x 10- 0.92 x 101 2.69 x 101 3.87 x 107 1.25 x 107 3.90 x 107 1.54 x 107 1.74 x 107 4 4 Compiled from [17]. "Most common valence given in parenthesis (); this is the value assumed in determining the plating constant C. For a different valence, compute the new Cby multiplying C value in the table by the most common valence and then dividing by the new valence.
Step by Step Solution
★★★★★
3.53 Rating (156 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


