Answered step by step
Verified Expert Solution
Question
1 Approved Answer
What reactor (CSTR or PFR) would you choose to maximize the total selectivity to D? The reactor will be operated isothermally at 200 C
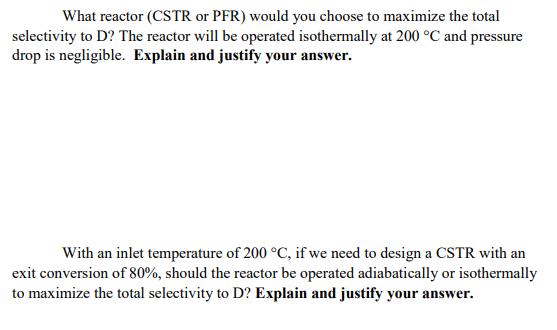
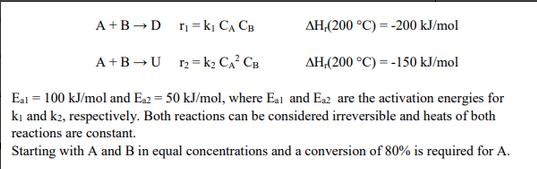
What reactor (CSTR or PFR) would you choose to maximize the total selectivity to D? The reactor will be operated isothermally at 200 C and pressure drop is negligible. Explain and justify your answer. With an inlet temperature of 200 C, if we need to design a CSTR with an exit conversion of 80%, should the reactor be operated adiabatically or isothermally to maximize the total selectivity to D? Explain and justify your answer. A+B D r = k CA CB AH (200 C)=-200 kJ/mol A+B U12 K2 CA CB AH,(200 C) -150 kJ/mol Eat 100 kJ/mol and Ea2 50 kJ/mol, where Eat and Eaz are the activation energies for ki and k2, respectively. Both reactions can be considered irreversible and heats of both reactions are constant. Starting with A and B in equal concentrations and a conversion of 80% is required for A.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


