Question
7.62L If 50.0 grams of H3PO4 react with 50.0 grams of MgCO3, then, according to the following reaction, determine the volume of carbon dioxide
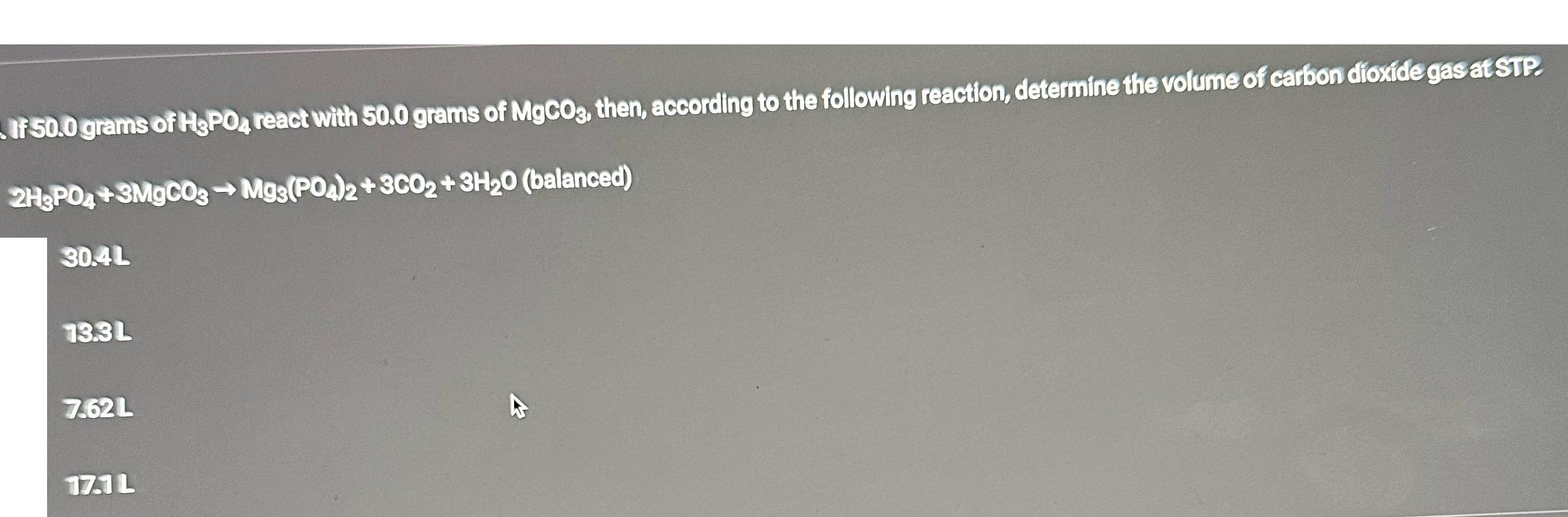
7.62L If 50.0 grams of H3PO4 react with 50.0 grams of MgCO3, then, according to the following reaction, determine the volume of carbon dioxide gas at STP. 2H3PO4+3MgCO3 Mg3(PO4)2+3CO2 + 3H20 (balanced) 30.4L 13.3L 17.1 L
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Intermediate Accounting
Authors: J. David Spiceland, James Sepe, Mark Nelson, Wayne Thomas
9th Edition
125972266X, 9781259722660
Students also viewed these Accounting questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



