Question
A piece of steel of unknown mass at a temperature of 98.2C is dropped into a beaker containing 780 g of water at a
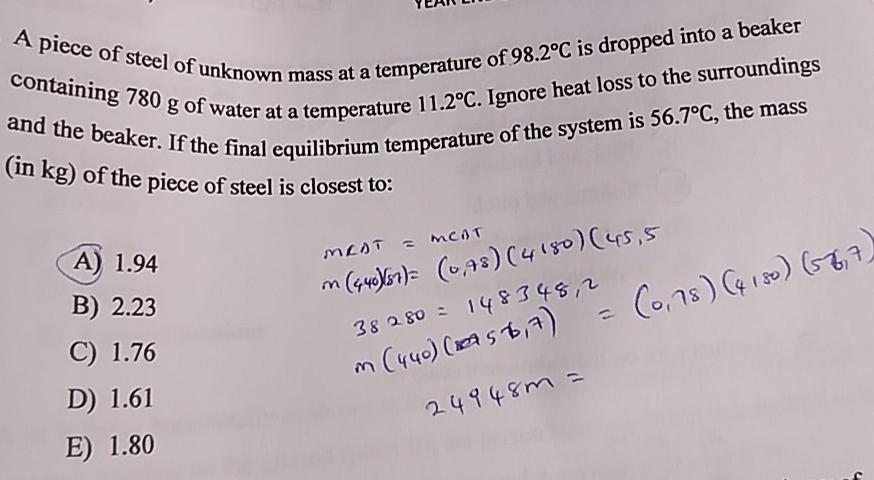
A piece of steel of unknown mass at a temperature of 98.2C is dropped into a beaker containing 780 g of water at a temperature 11.2C. Ignore heat loss to the surroundings and the beaker. If the final equilibrium temperature of the system is 56.7C, the mass (in kg) of the piece of steel is closest to: A) 1.94 B) 2.23 C) 1.76 D) 1.61 E) 1.80 MEAT = MCAT M (440)(87)= (0,78) (4180) (45,5 m = (0,78) (4180) (567) 38280 = 148348,2 (440) (2756,7) 249483=
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Chemical Principles
Authors: Steven S. Zumdahl, Donald J. DeCoste
7th edition
9781133109235, 1111580650, 978-1111580650
Students also viewed these Physics questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



