Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A plant produces 8640 tonnes per day (100 kg/s) of titanium dioxide pigment which must be 99.9 per cent pure when dried. The pigment
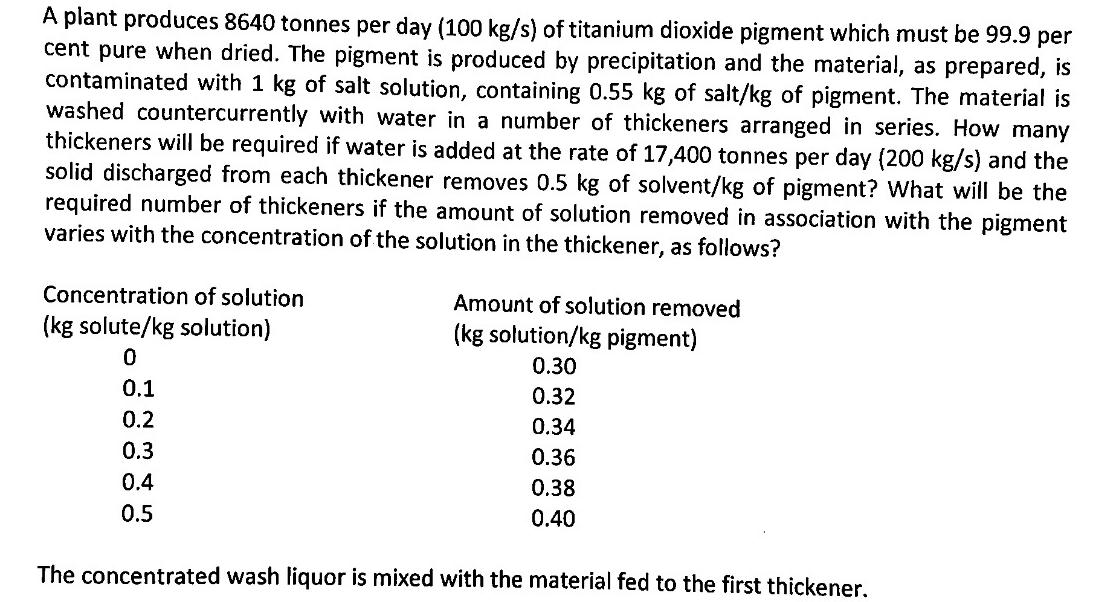
A plant produces 8640 tonnes per day (100 kg/s) of titanium dioxide pigment which must be 99.9 per cent pure when dried. The pigment is produced by precipitation and the material, as prepared, is contaminated with 1 kg of salt solution, containing 0.55 kg of salt/kg of pigment. The material is washed countercurrently with water in a number of thickeners arranged in series. How many thickeners will be required if water is added at the rate of 17,400 tonnes per day (200 kg/s) and the solid discharged from each thickener removes 0.5 kg of solvent/kg of pigment? What will be the required number of thickeners if the amount of solution removed in association with the pigment varies with the concentration of the solution in the thickener, as follows? Concentration of solution (kg solute/kg solution) 0 0.1 0.2 0.3 0.4 0.5 Amount of solution removed (kg solution/kg pigment) 0.30 0.32 0.34 0.36 0.38 0.40 The concentrated wash liquor is mixed with the material fed to the first thickener.
Step by Step Solution
There are 3 Steps involved in it
Step: 1
This problem involves the countercurrent washing of titanium dioxide pigment with water in a series ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


