Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A pressure line supplies a gas A and is connected to an adiabatic piston-cylinder system as shown below. The left, connected chamber is filled
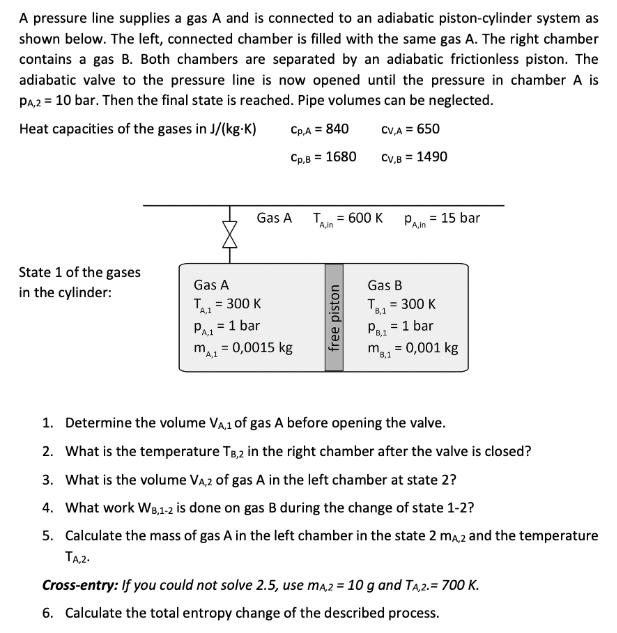
A pressure line supplies a gas A and is connected to an adiabatic piston-cylinder system as shown below. The left, connected chamber is filled with the same gas A. The right chamber contains a gas B. Both chambers are separated by an adiabatic frictionless piston. The adiabatic valve to the pressure line is now opened until the pressure in chamber A is PA,2 = 10 bar. Then the final state is reached. Pipe volumes can be neglected. Heat capacities of the gases in J/(kg-K) State 1 of the gases in the cylinder: Gas A Gas A = 300 K PA = 1 bar A.1 m = 0,0015 kg A,1 CpA = 840 CV,A= 650 CpB = 1680 CV,B=1490 T. A,in = 600 K Pain 15 bar free piston Gas B PB.1 = 300 K = 1 bar m = 0,001 kg 8,1 1. Determine the volume VA,1 of gas A before opening the valve. 2. What is the temperature TB,2 in the right chamber after the valve is closed? 3. What is the volume VA,2 of gas A in the left chamber at state 2? 4. What work W8,1-2 is done on gas B during the change of state 1-2? 5. Calculate the mass of gas A in the left chamber in the state 2 mA,2 and the temperature TA,2. Cross-entry: If you could not solve 2.5, use mA,2 = 10 g and TA,2.= 700 K. 6. Calculate the total entropy change of the described process.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


