Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A process utilizes the reaction of 2-chloro-2-methylpropane (C,H,CI) with an aqueous solution of sodium hydroxide (NaOH) to produce 2-methylpropene (C,H,), along with sodium chloride
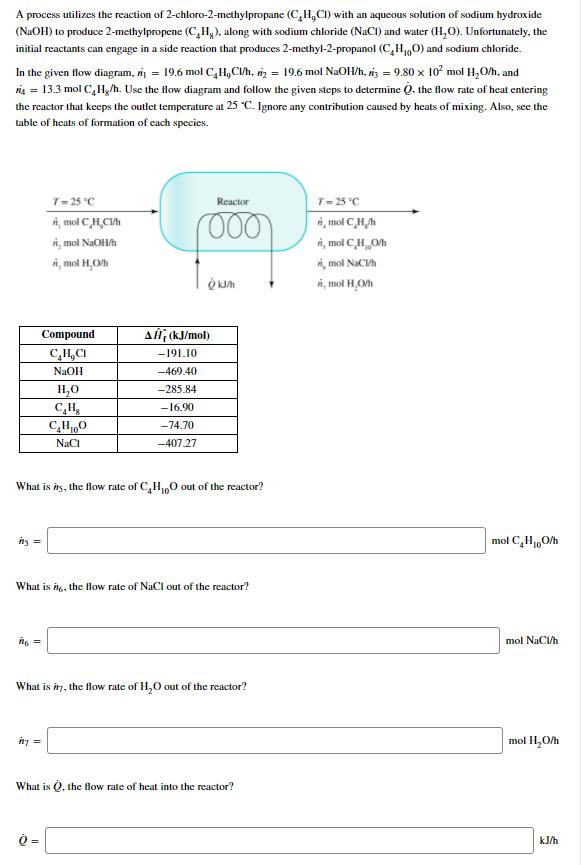
A process utilizes the reaction of 2-chloro-2-methylpropane (C,H,CI) with an aqueous solution of sodium hydroxide (NaOH) to produce 2-methylpropene (C,H,), along with sodium chloride (NaCl) and water (H2O). Unfortunately, the initial reactants can engage in a side reaction that produces 2-methyl-2-propanol (CHO) and sodium chloride. In the given flow diagram, i=19.6 mol C,H,Cl/h, 2 = 19.6 mol NaOH/h, 3 = 9.80 x 102 mol HO/h, and r4 = 13.3 mol C4H/h. Use the flow diagram and follow the given steps to determine Q, the flow rate of heat entering the reactor that keeps the outlet temperature at 25 C. Ignore any contribution caused by heats of mixing. Also, see the table of heats of formation of each species. T-25C i, mol C,H,Ch ii, mol NaOH/h i, mol H,O/h Reactor 000 T-25C Amol CH i, mol CHO/h i, mol NaCl/h i, mol H,O/h Compound CHCI NaOH Al (kJ/mol) -191.10 -469.40 HO -285.84 CH -16.90 CHO -74.70 NaCl -407.27 What is its, the flow rate of C4H100 out of the reactor? ng = What is it, the flow rate of NaCl out of the reactor? n = mol CHO/h mol NaCl/h What is in, the flow rate of HO out of the reactor? mol HO/h in= What is Q, the flow rate of heat into the reactor? Q= kJ/h
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


