Question
A propane BBQ combusts propane as shown below: C3H8 (g) + 5 O2(g) 3 CO2 (g) + 4 HO (1) ArH=-2220 kJ/mol What mass
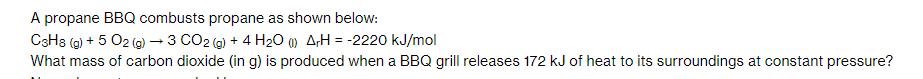
A propane BBQ combusts propane as shown below: C3H8 (g) + 5 O2(g) 3 CO2 (g) + 4 HO (1) ArH=-2220 kJ/mol What mass of carbon dioxide (in g) is produced when a BBQ grill releases 172 kJ of heat to its surroundings at constant pressure?
Step by Step Solution
There are 3 Steps involved in it
Step: 1
Calculating the mass of carbon dioxide produced Step 1 Determine the enthalpy change per gram of CO2 ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Elementary Principles of Chemical Processes
Authors: Richard M. Felder, Ronald W. Rousseau
3rd Edition
978-0471687573, 9788126515820, 978-0-471-4152, 0471720631, 047168757X, 8126515821, 978-0471720638
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



