Answered step by step
Verified Expert Solution
Question
1 Approved Answer
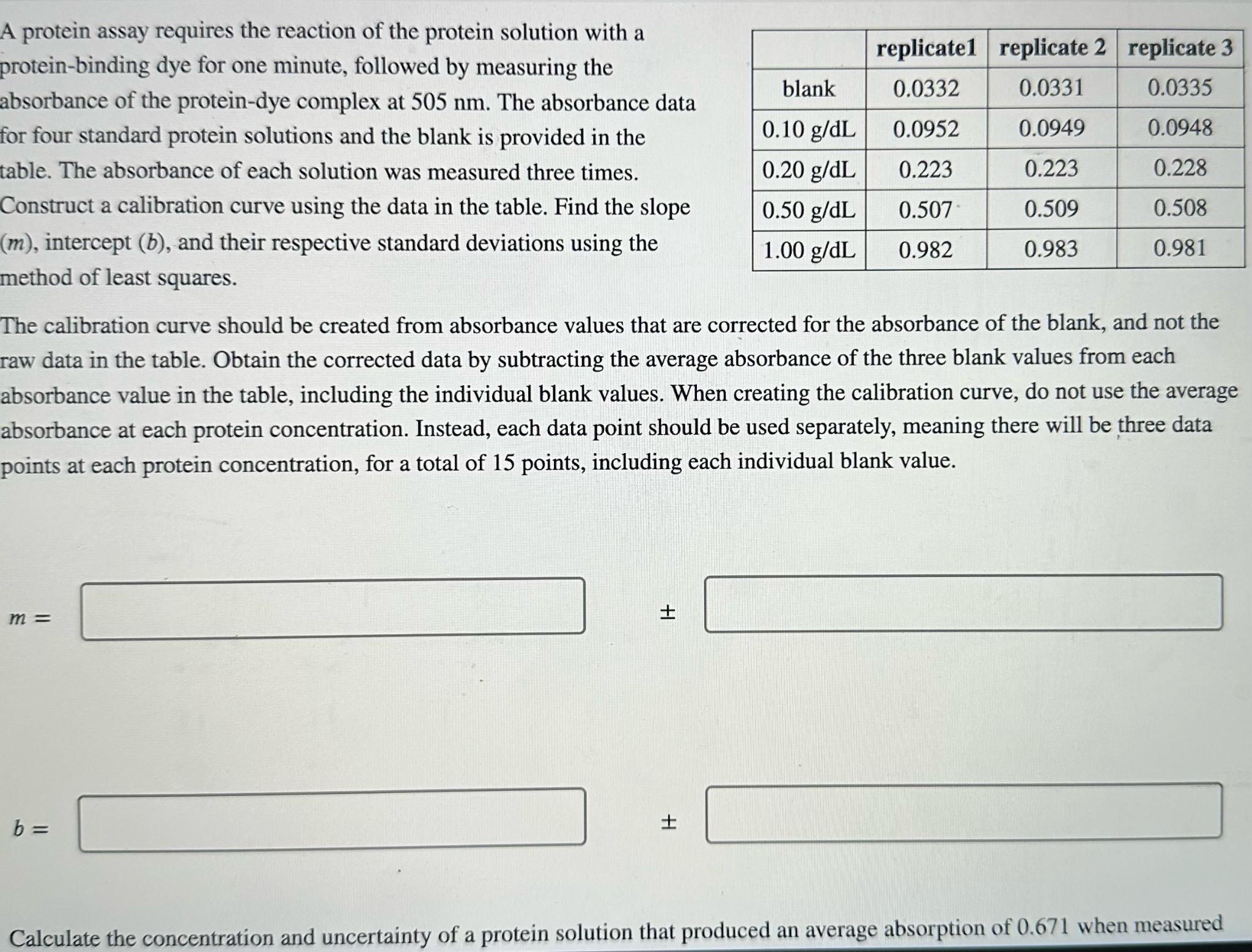
A protein assay requires the reaction of the protein solution with a protein - binding dye for one minute, followed by measuring the absorbance of
A protein assay requires the reaction of the protein solution with a proteinbinding dye for one minute, followed by measuring the absorbance of the proteindye complex at The absorbance data for four standard protein solutions and the blank is provided in the table. The absorbance of each solution was measured three times. Construct a calibration curve using the data in the table. Find the slope intercept and their respective standard deviations using the method of least squares.
tablereplicatereplicate replicate blank
The calibration curve should be created from absorbance values that are corrected for the absorbance of the blank, and not the raw data in the table. Obtain the corrected data by subtracting the average absorbance of the three blank values from each absorbance value in the table, including the individual blank values. When creating the calibration curve, do not use the average absorbance at each protein concentration. Instead, each data point should be used separately, meaning there will be three data points at each protein concentration, for a total of points, including each individual blank value.
Calculate the concentration and uncertainty of a protein solution that produced an average absorption of when measured
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


