Answered step by step
Verified Expert Solution
Question
1 Approved Answer
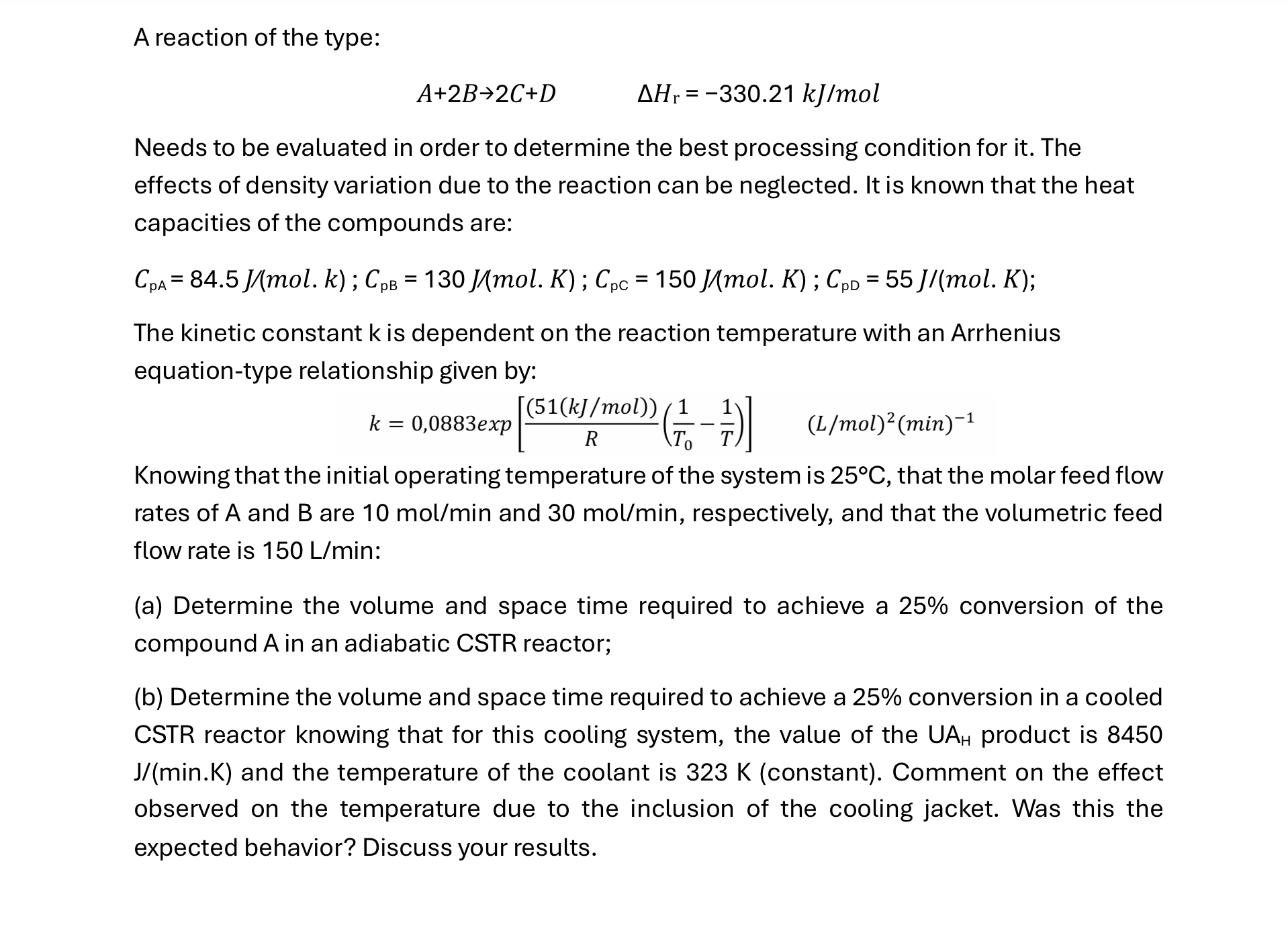
A reaction of the type: A + 2 B 2 C + D , H r = - 3 3 0 . 2 1 k
A reaction of the type:
Needs to be evaluated in order to determine the best processing condition for it The effects of density variation due to the reaction can be neglected. It is known that the heat capacities of the compounds are:
:;
The kinetic constant is dependent on the reaction temperature with an Arrhenius equationtype relationship given by:
exp
Knowing that the initial operating temperature of the system is that the molar feed flow rates of A and are and respectively, and that the volumetric feed flow rate is :
a Determine the volume and space time required to achieve a conversion of the compound in an adiabatic CSTR reactor;
b Determine the volume and space time required to achieve a conversion in a cooled CSTR reactor knowing that for this cooling system, the value of the product is minK and the temperature of the coolant is constant Comment on the effect observed on the temperature due to the inclusion of the cooling jacket. Was this the expected behavior? Discuss your results.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


