Question
A rigid, well-insulated tank contains air. A partition in the tank separates 12 ft of air at 14.7 lbf/in., 40F from 10 ft of
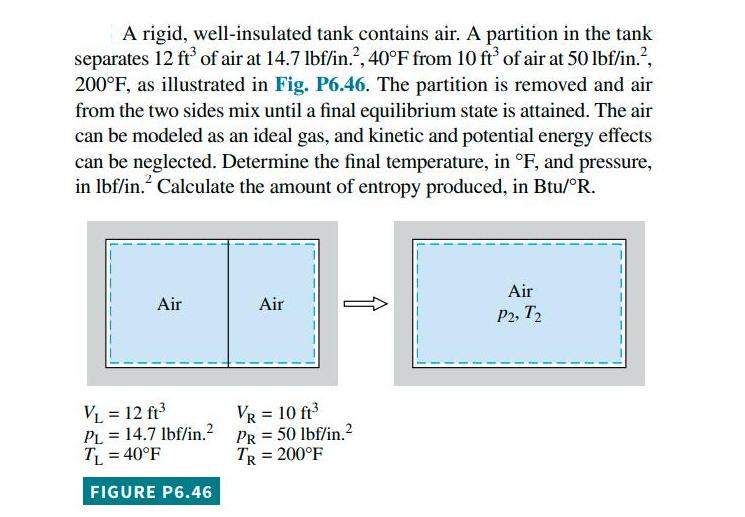
A rigid, well-insulated tank contains air. A partition in the tank separates 12 ft of air at 14.7 lbf/in., 40F from 10 ft of air at 50 lbf/in., 200F, as illustrated in Fig. P6.46. The partition is removed and air from the two sides mix until a final equilibrium state is attained. The air can be modeled as an ideal gas, and kinetic and potential energy effects can be neglected. Determine the final temperature, in F, and pressure, in lbf/in. Calculate the amount of entropy produced, in Btu/R. Air VL = 12 ft PL = 14.7 lbf/in.2 TL = 40F - FIGURE P6.46 Air VR = 10 ft PR = 50 lbf/in. TR = 200F Air P2, T
Step by Step Solution
3.29 Rating (158 Votes )
There are 3 Steps involved in it
Step: 1
Before mixing For left partition VL 12 ft PL 147 lbfin 21168 lbfft TL 40F 500R For right partition V...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Thermodynamics for Engineers
Authors: Kenneth A. Kroos, Merle C. Potter
1st edition
1133112862, 978-113311286
Students also viewed these Electrical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



